- DL manuals
- Abbott
- Measuring Instruments
- i-STAT 1
- System Manual
Abbott i-STAT 1 System Manual
Summary of i-STAT 1
Page 1
I-stat ® 1 system manual rev. Date: 07-mar-13 art: 714336-01m.
Page 2
Patents: www.Abbott.Us/patents symbol technologies corporation is the owner of us patent no. 5,532,469. Trademarks i-stat is a registered trademark of the abbott group of companies in various jurisdictions. Windows is a registered trademark of microsoft corporation. Art: 714336-01m rev. Date: 07-mar...
Page 3
Please ensure that the contents of your system manual are complete and up to date. In the event that your system manual does not contain the current configuration, it is recommended that you contact your i-stat support provider. As of may 2017, your i-stat p ® p 1 system manual should be configured ...
Page 4
Potassium ......................................................................................... 714174-01o chloride ............................................................................................. 714175-01o urea nitrogen/bun ............................................................
Page 5: Contents
Art: 714362-01v rev. Date: 27-apr-17 i contents introduction .................................................................................................... 1 - 1 this manual ...........................................................................................................................
Page 6
Ii art: 714362-01v rev. Date: 27-apr-17 i-stat cartridge ............................................................................................... 3 - 1 contents .......................................................................................................................................
Page 7
Art: 714362-01v rev. Date: 27-apr-17 iii ir link .................................................................................................................................................. 8 - 3 lis/his interface ...................................................................................
Page 8
Iv art: 714362-01v rev. Date: 27-apr-17 controls for act cartridges ................................................................................................................ 14 - 9 controls for pt/inr cartridges ....................................................................................
Page 9
Art: 714362-01v rev. Date: 27-apr-17 v theory theory .................................................................................................................. 20 - 1 analyzer functions .............................................................................................................
Page 10
Vi art: 714362-01v rev. Date: 27-apr-17 cartridge and test information cartridge and test information sodium potassium chloride bun/urea glucose hematocrit/hemoglobin ionized calcium po 2 ph pco 2 total carbon dioxide/tco 2 creatinine lactate celite act kaolin act prothrombin time pt/inr cardiac tro...
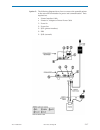
Page 11: Introduction
Introduction 1 art: 714363-01w rev. Date: 21-mar-14 1-1 this manual this manual describes the i-stat 1analyzer and the central data station software. Related sections are grouped behind tabs. Intended use the i-stat 1analyzer is intended for use with i-stat cartridges for the in vitro quantification...
Page 12
1-2 art: 714363-01w rev. Date: 21-mar-14 selection of components the selection of system components is dependent on factors unique to each facility such as: types of tests to be performed number of testing sites number of tests per site system administration requirements summary of the procedure to ...
Page 13
Art: 714363-01w rev. Date: 21-mar-14 1-3 data management test records can be transmitted to the data manager where they can be printed and/or transmitted to the laboratory information system or hospital information system. An optional portable printer enables the operator to print results at the poi...
Page 14
1-4 art: 714363-01w rev. Date: 21-mar-14 symbol definition manufacturer's lot number or batch code. The lot number or batch will appear adjacent to this symbol. Catalog number, list number, or reference number. The number adjacent to this symbol is used to reorder the product. Serial number. The ser...
Page 15
Art: 714363-01w rev. Date: 21-mar-14 1-5 symbol definition noteconcerningbatteries:thefollowinginformationisapplicabletoeea(european economicarea)countries:thedirective2006/66/ecrequiresseparatecollectionof spent batteries. You are requested to dispose those batteries referred to on page 2-3 in acco...
Page 16
1-6 art: 714363-01w rev. Date: 21-mar-14 symbol the following symbols are used on the i-stat portable clinical analyzer keypad dis key used to activate the display. Ent key used to enter information. Prt key used to print a test record. Clr key used to clear an incorrect entry. Symbol the following ...
Page 17
Art: 714363-01w rev. Date: 21-mar-14 1-7 symbol test angap anion gap so2 oxygen saturation ctni cardiac troponin i ck-mb creatine kinase mb isoenzyme bnp b-type natriuretic peptide warranty abbottpointofcareinc.Warrantsthismedicalproduct(excludingdisposableor consumablesupplies)againstdefectsinmater...
Page 18
1-8 art: 714363-01w rev. Date: 21-mar-14.
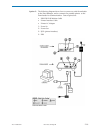
Page 19: I-Stat 1 Analyzer
I-stat 1 analyzer 2 art: 714364-01o rev. Date: 26-feb-16 2-1 introduction the i-stat 1 analyzer is used in conjunction with i-stat cartridges for the simultaneous quantitative determination of specific analytes in whole blood. Refer to the cartridge and test information section of this manual for in...
Page 20
2-2 art: 714364-01o rev. Date: 26-feb-16 software battery compartment the battery compartment is located at the display end of the analyzer next to the laser barcode scanner window. The procedure for changing disposable and rechargeable batteries can be found in the routine care of the analyzer and ...
Page 21
Art: 714364-01o rev. Date: 26-feb-16 2-3 disposable batteries the analyzer requires two 9-volt lithium batteries. The lifetime for a set of batteries is mainly dependent on the mix of cartridges in use. Cartridges that require thermal control consume more energy because of heating. Coagulation and i...
Page 22
2-4 art: 714364-01o rev. Date: 26-feb-16 additional power a lithium battery inside the analyzer maintains the clock/calendar and customization profile. This battery should last seven years. Cartridges and the electronic simulator are inserted into the analyzer through the cartridge port on the keypa...
Page 23
Art: 714364-01o rev. Date: 26-feb-16 2-5 infrared communication window the infrared communication window provides the analyzer with two-way communication to the central data station via a downloader, allows analyzer- to-analyzer software updates, and allows analyzer-to-printer communication for prin...
Page 24
2-6 art: 714364-01o rev. Date: 26-feb-16 data entry data that can be scanned into the analyzer or entered via the keypad include: operator id patient id, proficiency id, or simulator id cartridge lot number control lot number cal ver kit lot number comment codes for patient and control results chart...
Page 25
Art: 714364-01o rev. Date: 26-feb-16 2-7 lcd display and backlight audible indicator the analyzer will beep to indicate: whenever a key is pressed. A successful barcode entry. Results are ready. A quality check message is displayed. The analyzer can be customized to disable beeping when a key is pre...
Page 26
2-8 art: 714364-01o rev. Date: 26-feb-16 keypad there are 19 keys located directly below the display. When using the keypad to enter information, the number of dashes in the data entry line will indicate how many characters can be entered on the line. The dash where the next entry will be placed wil...
Page 27
Art: 714364-01o rev. Date: 26-feb-16 2-9 i-stat 1 menu tree there are two main menus: the test menu and the administration menu. . Test menu administration menu 1- last result 1. Analyzer status temp 2- i-stat cartridge pressure battery uses serial clew version custom stored records total unsent 2- ...
Page 28
2-10 art: 714364-01o rev. Date: 26-feb-16 test menu administration menu overview the test menu is displayed when the analyzer is turned on using the on/off key. The options are: 1 - last result 2 - i-stat cartridge option 2 is used for testing patient samples. Note: if the handheld is customized to ...
Page 29
Art: 714364-01o rev. Date: 26-feb-16 2-11 the analyzer status screen contains information about the condition or “status” of the analyzer. Fresh readings are made whenever this option is selected. Temp room temperature. Pressure barometric pressure. Battery battery voltage. Uses total number of cart...
Page 30
2-12 art: 714364-01o rev. Date: 26-feb-16 7 - list records are listed with cartridge type, date and time of test, patient id, control lot, proficiency id, or cal ver lot and test level as applicable. Any number of test records can be selected for viewing or printing using the number keys. Pressing t...
Page 31
Art: 714364-01o rev. Date: 26-feb-16 2-13 customization analyzers can be customized for site-specific testing characteristics and requirements. A complete list of customizable parameters and their default values can be found in the customization section. An analyzer can be customized via the keypad ...
Page 32
2-14 art: 714364-01o rev. Date: 26-feb-16 viewing the customization profile select 4- customization from the administration menu, select 1- view then select from the customization menu: 1 - analyzer 2 - id entry 3 - patient tests 4 - qc tests 5 - results select a category to review. Use the ← and → ...
Page 33
Art: 714364-01o rev. Date: 26-feb-16 2-15 third page code 39 check digit truncate first truncate last operator list not certified action not in list action fourth page warn user print id 2 – patient id first page minimum length maximum length repeat id id recall manual entry second page code i2of5 c...
Page 34
2-16 art: 714364-01o rev. Date: 26-feb-16 changing the profile to customize via the handheld keypad, select 4- customization from the administration menu, then select 2- change. If the handheld has already been customized with a password, enter the password. If not, press the enter key. (it is recom...
Page 35
Art: 714364-01o rev. Date: 26-feb-16 2-17 2 - id entry 1 – operator id first page minimum length maximum length repeat id manual entry code i2of5 second page code 128 ean-8, ean-13 codabar code 93 code 39 third page code 39, check digit truncate first truncate last print id 2 – patient id first page...
Page 36
2-18 art: 714364-01o rev. Date: 26-feb-16 2 – cartridge qc pass/fail method comment code in range comment code out of range result format apoc fluid lot scan only 5 - results 1 – units and ranges 2 – options first page decimal separator test selection hematocrit base excess act-c second page act-k p...
Page 37
Art: 714364-01o rev. Date: 26-feb-16 2-19 set clock if the analyzer is customized with a password, the set clock function will be password protected. If a password has not been assigned, pressing the enter key will display the time and date screen. Use the arrow keys to move the cursor to the digit ...
Page 38
2-20 art: 714364-01o rev. Date: 26-feb-16 laser barcode scanner the barcode scanner is used to scan barcode information into the analyzer. Parameters that can be entered into the analyzer via the scanner include: operator and patient ids, control and cartridge lot numbers, comment codes and patient ...
Page 39
Art: 714364-01o rev. Date: 26-feb-16 2-21 caution do not open the analyzer. The analyzer may only be opened by factory authorized service personnel. Class 2 laser radiation when open; do not stare into the laser aperture or the laser beam, or point the laser beam at other persons. Use of controls, a...
Page 40
2-22 art: 714364-01o rev. Date: 26-feb-16 startup messages quality check messages if the analyzer detects a problem during power on, a quality check message will be displayed indicating the action that must be taken before testing can begin. A quality check message will also be displayed and testing...
Page 41: I-Stat Cartridge
3-1 i-stat cartridge rev. Date: 11-mar-14 art: 714365-01g 3 contents the unit-use disposable cartridge contains many of the subassemblies typically found in complex laboratory systems. Microfabricated thin film electrodes or sensors are assembled in unit-use cartridges containing: • calibrant soluti...
Page 42: Eg7+
3-2 art: 714365-01g rev. Date: 11-mar-14 sample handling system part function sensor channel the sensor channel directs the sample from the sample chamber to the sensors. An extension of this channel becomes a waste chamber to receive the calibrant solution, if applicable, as it is displaced by the ...
Page 43: Ec8+
3-3 rev. Date: 11-mar-14 art: 714365-01g standardization and calibration standardization is the process by which a manufacturer establishes “true” values for representative samples. The sensors in the i-stat cartridges are standardized against plasma methods used by major laboratory systems or, for ...
Page 44
3-4 art: 714365-01g rev. Date: 11-mar-14 storage conditions the main supply of cartridges should be stored at 2-8°c (35-46°f). Cartridges must be at room temperature before removing them from their pouches. Allow 5 minutes for an individual cartridge and one hour for a box of cartridges to come to r...
Page 45
Art: 714366-01c rev. Date: 31-jan-12 4 precision pcx and pcx™ plus blood glucose test strips upon installation of the april 2012 software update, the glucose test strip port functionality will be permanently disabled on all i-stat 1 handhelds. Note: the remaining technical information regarding the ...
Page 46
2 art: 714366-01c rev. Date: 31-jan-12.
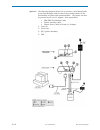
Page 47: Electronic Simulator
Electronic simulator 5 art: 714367-01c rev. Date: 25-aug-11 1 the external electronic simulator is a stable electronic device, which is inserted into the cartridge port. The test cycle for the external electronic simulator is about 60 seconds. (the test cycle for the internal simulator is shorter be...
Page 48
2 art: 714367-01c rev. Date: 25-aug-11 even when the internal electronic simulator is enabled, an external electronic simulator is needed: • tovalidateaninternalsimulatorfailure. • toresettheinternalsimulatorscheduleifa simulator test might interrupt testing, such as in a cvor. Note: cvor = cardiova...
Page 49: I-Stat 1 Downloader
Art: 714368-01j rev. Date: 02-aug-12 6 i-stat 1 downloader function the downloader converts infrared transmissions of test records from the analyzer to electrical form and transmits (uploads) them to the data manager. The downloader also converts electrical signals from the central data station to i...
Page 50
6 - 2 art: 714368-01j rev. Date: 02-aug-12 specifications power supply specification downloader and downloader/recharger input 100 - 240v~ 47 - 63 hz .9 - .5a output 12v 3a max * recharge feature cannot be used in this configuration. Specification downloader downloader/recharger size 5.25in (13.3cm)...
Page 51
6 - 3 art: 714368-01j rev. Date: 02-aug-12 downloader/ recharger indicator leds analyzer battery led (near top of downloader/recharger) off no rechargeable battery blinking red fast charge pending solid red fast charging solid green trickle charging s pare b attery (near middle of downloader/recharg...
Page 52
6 - 4 art: 714368-01j rev. Date: 02-aug-12 transmitting data from downloader to the data manager transmitting data from downloader / recharger to the data manager to transmit data through a downloader/recharger, place the analyzer in the downloader/recharger’s cradle. When properly aligned, the blue...
Page 53
6 - 5 art: 714368-01j rev. Date: 02-aug-12 troubleshooting the analyzer displays “waiting to send” until communication is established with the central data station. When communication is established the message changes to “communication in progress” and the arrows circle until upload is complete. If...
Page 54
6 - 6 art: 714368-01j rev. Date: 02-aug-12 charging rechargeable battery in external recharge compartment placing a rechargeable battery into the recharging compartment will automatically initiate trickle recharging. The indicator light near the recharging compartment will be green when a rechargeab...
Page 55: Technical Bulletin
Abbott point of care inc. • abbott park, il 60064 • usa art: 728690-01f rev. Date: 14-mar-14 technical bulletin i-stat ® the i-stat ® 1 downloader/recharger (model number drc-300) overview this technical bulletin describes the instructions for using the new i-stat ® 1 downloader/recharger (drc), whi...
Page 56
Art: 728690-01f rev. Date: 14-mar-14 2 table of contents section title page number identification of the new i-stat 1 downloader/recharger 2 specifications of the drc-300 3 power supply specifications 3 drc indicator leds 3 power requirements 4 cautions 4 running cartridges in a handheld docked in t...
Page 57
3 rev. Date: 14-mar-14 art: 728690-01f specifications of the drc-300 specification size 4.12in (10.4cm) wide 9.60in (24.4cm) long 5.00in (12.7cm) high weight 1.2 lbs (0.55kg) power ac-dc power adapter input 12vdc operating temperature 0 - 40ºc 32 -104ºf storage temperature -20 - 50ºc -4 - 122ºf poll...
Page 58
Art: 728690-01f rev. Date: 14-mar-14 4 spare battery (near middle of drc) off no rechargeable battery green trickle charging power requirements the drc requires one power outlet. The drc must be used with the ac power supply adapter supplied with the drc. Using the y-splitter cable, the drc power su...
Page 59
5 rev. Date: 14-mar-14 art: 728690-01f transmitting data from the drc to the data manager 1. Place handheld in the drc’s cradle. If properly aligned, the blue proximity light will turn on and a “waiting to send” message will be displayed on the handheld until communication is estab- lished with the ...
Page 60
Art: 728690-01f rev. Date: 14-mar-14 6 charging a rechargeable battery while installed in the handheld placing a handheld containing the rechargeable battery in the drc will automatically initiate charging of the rechargeable battery. The indicator light on top of the drc will be: • green (trickle c...
Page 61
7 rev. Date: 14-mar-14 art: 728690-01f note: if the “enable network communications” box is not checked then network communication is not enabled at your facility and these actions do not apply to you. 5. At the “ maximum number of simultaneous network connections” selection box, increase the number ...
Page 62
Art: 728690-01f rev. Date: 14-mar-14 8 3. Windows 7: change the pc network configuration to detect the drc. On the pc, click start → control panel → network and internet (if shown, skip if not) → network and shar- ing center → view network status and tasks. Windows xp: change the pc network configur...
Page 63
9 rev. Date: 14-mar-14 art: 728690-01f 6. In the general tab, record all internet protocol (tcp/ip) properties for later use. 7. Select the “use the following ip address” radio button and input the following information: • ip address: 192.168.1.8 • subnet mask: 255.255.255.0 • default gateway: 192.1...
Page 64
Art: 728690-01f rev. Date: 14-mar-14 10 if the webpage does not appear, the password to the drc has been forgotten, or the drc ip address settings are unknown, it is possible to temporarily* reset all drc settings back to factory default. 1. Connect the network cable between the pc and the drc, if n...
Page 65
11 rev. Date: 14-mar-14 art: 728690-01f 11. Under “current settings”, click configure. The configure communication settings page will appear. 12. Determine the following site-specific information for this drc: • ip address of the drc • subnet mask • default gateway address • ip address of the data m...
Page 66
Art: 728690-01f rev. Date: 14-mar-14 12 after completion, the following screen will be displayed. 17. If additional drcs require configuration, connect the next drc to the pc per step 2 and apply power to the drc and repeat steps 9 through 16. Otherwise, proceed to step 18. Restoring the pc’s networ...
Page 67
13 rev. Date: 14-mar-14 art: 728690-01f connecting and wiring the drc for network communication the following diagram shows how to connect the portable printer to the drc and the drc to the net- work for communication to the data manager. The required parts are: • printer interface cable • downloade...
Page 68
Art: 728690-01f rev. Date: 14-mar-14 14 configuring the i-stat 1 drc for serial operation to install the usb drivers for the drc, it is necessary to be logged into a windows pc with administrator rights. Note: if internet access is not available, obtain the i-stat 1 d/r usb driver cd-rom (abbott l/n...
Page 69
15 rev. Date: 14-mar-14 art: 728690-01f 6. Another “found new hardware wizard” will appear automatically. Select the “yes, this time only” radio button and click next to continue. 7. Select the “install a software automatically (recommended)” radio button as previously illus- trated and click next. ...
Page 70
Art: 728690-01f rev. Date: 14-mar-14 16 13. Using the drop down menu, set the “bits per second” to 38400. Other drop down menus should remain as the default. 14. Click advance settings. Using the drop down menu, change the port number to the lowest available number. Determine availability by viewing...
Page 71
17 rev. Date: 14-mar-14 art: 728690-01f 5. Apply power to the drc. The following notifications as shown below should be displayed. 6. Select start → settings → control panel → performance and maintenance (if listed) → system to launch the “system properties” dialog box. Select the hardware tab, and ...
Page 72
Art: 728690-01f rev. Date: 14-mar-14 18 8. Right click on the “usb serial port” device entry and select properties. A “usb serial port properties” dialog box will open. Select the port settings tab. 9. Using the drop down menu, set the “bits per second” to 38400. Other drop down menus should remain ...
Page 73
19 rev. Date: 14-mar-14 art: 728690-01f pc 1 2 3 power in rj12 (printer interface) usb y-splitter cable * * if using a martel printer, use the power supply that came with the martel printer and not the y-splitter cable provided with the drc. Connecting and wiring the i-stat 1 drc for serial communic...
Page 74
Art: 728690-01f rev. Date: 14-mar-14 20 note 1.1: this utility is only compatible with windows xp and higher. Note 1.2: other ftdi usb device drivers with vendor id: 0403 and productid: 6001 will be uninstalled using the same uninstallation process. •instructionsforthewindowsxpusbuninstallerutilityc...
Page 75: Portable Printer
Art: 714369-01k rev. Date: 06-apr-15 7 7 - 1 portable printer martel printer overview specifications dimensions height: 64mm width: 135mm depth: 130mm weight 425g (approx.) power 1. 4.8v nickle metal hydride battery pack. 2. Power adapter for ac outlet 3. Downloader communication link 1. Infra-red 2...
Page 76
7 - 2 art: 714369-01k rev. Date: 06-apr-15 supplies provided with printer power the printer is turned on using the switch on its left side. When the printer is on, the power led will be green. The plug for the ac adaptor is also on the left side. For printer serial numbers below 240223657, the recha...
Page 77
7 - 3 art: 714369-01k rev. Date: 06-apr-15 squeeze cup lid to gain access to paper roll paper position of paper roll in printer when removing a printout from the printer, pull the printout toward the front of the printer and tear from one side to the other across the serrated edge. Using serrated ed...
Page 78
7 - 4 art: 714369-01k rev. Date: 06-apr-15 printing many results select 2 – data review from the administration menu on the analyzer, then select 7 – list. Use the arrow keys to page up and down through the pages of stored results. Press the numbered key for each test record to be printed. To desele...
Page 79
7 - 5 art: 714369-01k rev. Date: 06-apr-15 troubleshooting printer not printing. Power led on and status led off: check that results are displayed or that results have been selected from list under data review. Check that that distance between analyzer and printer, if printing directly from the anal...
Page 80: The I-Stat Printer
7 - 6 art: 714369-01k rev. Date: 06-apr-15 the i-stat printer overview this section describes the instructions for using the new i-stat printer, which is used to print results from all models of the i-stat 1 analyzer (handheld). Note: this printer cannot be used with the i-stat portable clinical ana...
Page 81
7 - 7 art: 714369-01k rev. Date: 06-apr-15 specifications dimensions height: 72.5mm width: 136mm depth: 120mm weight 500g (approx.) power 1. 4.8v nimh rechargeable battery pack 2. Power adaptor for ac outlet communication link 1. Infra-red 2. Rj12 paper 5.7cm thermal buttons 1. On/off 2. Paper feed ...
Page 82
7 - 8 art: 714369-01k rev. Date: 06-apr-15 i-stat printer kit components and accessories the following individual components are included in the i-stat printer kit: 1. I-stat printer 2. Ac adapter 3. Power cord 4. Rechargeable battery 5. One roll of printer paper (not shown below) orderable componen...
Page 83
7 - 9 art: 714369-01k rev. Date: 06-apr-15 i-stat printer paper printer paper may be ordered along with other supplies for the i-stat system (abbott list # 06f17-11): the status indicator will illuminate to indicate the print status: ready: green out of paper: orange error: red paper for the i-sta...
Page 84
7 - 10 art: 714369-01k rev. Date: 06-apr-15 the i-stat printer can be turned on and off by pressing the power button. When the printer is on, the power indicator will be illuminated: power ok: green battery low: orange battery empty: red if the printer is inactive for >60 seconds, it will automati...
Page 85
7 - 11 art: 714369-01k rev. Date: 06-apr-15 5. Assure proper connector alignment as shown. 6. Slide the connector onto the three metal connector pins. 7. Once the wires are connected, place the battery portion of the pack into the rectangular compartment. Make sure the wires are not under the batter...
Page 86
7 - 12 art: 714369-01k rev. Date: 06-apr-15 8. Slide the battery door back onto the compartment until it closes and locks into place. 9. Turn the printer over, plug it back into the ac power adapter, and charge the new battery in the printer for a minimum of 3 hours before use. Note: if the recharge...
Page 87
7 - 13 art: 714369-01k rev. Date: 06-apr-15 connecting the i-stat printer to a downloader or down- loader/recharger the downloader programming and wiring section of the i-stat 1 system manual describes directions for connecting the i-stat printer to a downloader or downloader/recharger. Note: the fo...
Page 88
7 - 14 art: 714369-01k rev. Date: 06-apr-15 printout contents name of test i-stat catridge type sample id patient id or quality test type and lot number of solution tested results results are printed with units as well as flags, reference ranges, and comment codes, if ap- plicable at patient tempera...
Page 89
7 - 15 art: 714369-01k rev. Date: 06-apr-15 • do not operate the printer without paper. • do not allow the power supply to become a trip hazard. • do not disturb the handheld or printer until printing is complete since this will interrupt the printout. If printing is interrupted, realign the printer...
Page 90
7 - 16 art: 714369-01k rev. Date: 06-apr-15 printer symptom recommmended action(s) printer is feeding paper, but nothing is printed. Check that the paper is feeding from under the roll. Printer is not printing and power indicator is red. Battery needs to be recharged. Printer power indicator does no...
Page 91: Technical Bulletin
Abbott point of care inc. • abbott park, il 60064 • usa : t r a 728730-01c rev. Date: 02-dec-11 technical bulletin i-stat ® . I-stat printer storage and battery check notice this technical bulletin provides supplemental instruction on i-stat printer storage and assessing i-stat printer rechargeable ...
Page 93: Data Management
Data management 8 art: 714370-01d rev. Date: 03-aug-12 8-1 introduction the i-stat system provides comprehensive data management capabilities to ensure that blood analysis results obtained at the patient bedside can be integrated into the hospital’s various information systems. The data manager comp...
Page 94
8-2 art: 714370-01d rev. Date: 03-aug-12 the data manager this is i-stat’s primary data management application. It supports all blood analysis instruments mentioned above via a combination of serial and/or network communications. Please see the “central data station 5” section of this system manual ...
Page 95
Art: 714370-01d rev. Date: 03-aug-12 8-3 archive records export records to ascii text files manage instruments manage operators manage inventory manage policy exceptions monitor operator competence monitor lis entry exceptions monitor download compliance downloader and downloader/ recharger the down...
Page 96
8-4 art: 714370-01d rev. Date: 03-aug-12 standard data management configuration connecting components there is only one option available for physically connecting remote downloaders, downloader/rechargers, and ir links to a data manager. That option is: ethernet connection the i-stat system connects...
Page 97: Customiza
Customiza tion 9 art: 714371-01f rev . Date: 25-mar -16 9-1 overview this section describes the parameters that can be customized for site-specific testing r equir ements and the factory default settings. For the pr ocedur e to customize using the central data station see the central data station se...
Page 98
9-2 art: 714371-01f rev . Date: 25-mar -16 analyzer customization options and default settings option description default comments language window language for text: english, japanese, german, italian, dutch, spanish, french, swedish, portuguese, danish, and finnish english unit set window reporting...
Page 99
Art: 714371-01f rev . Date: 25-mar -16 9-3 preference window: for i nstrument o ptions option description default comments password 0-5 digit password to access set clock, the change function in customization, and utility under the administration menu. No password password protection for the set clo...
Page 100
9-4 art: 714371-01f rev . Date: 25-mar -16 preference window: for o perator and p atient id o ptions option description default comments operator id minimum and maximum allowed operator id length (scanned or manually entered) min = 0 max = 15 if operator ids are a fixed length, the min. And max. Set...
Page 101
Art: 714371-01f rev . Date: 25-mar -16 9-5 preference window: for t est o ptions option description default comments auto-chart presentation if enabled, the chart page will be displayed automatically. Not enabled: operator must press the → key to display the chart page. If any information on the cha...
Page 102
9-6 art: 714371-01f rev . Date: 25-mar -16 preference window: for c artridge qc – e lectronic qc s ettings option description default comments external simulator schedule options are off (no prompt), an interval of specified hours (1 to 65535 hours), or an interval of specified patient tests (up to ...
Page 103
Art: 714371-01f rev . Date: 25-mar -16 9-7 control test settings if the system administrator wants users to enter a comment code when liquid qc results are in-range, out-of-range, or under both situations, they would check the appropriate box and then use the drop down menu to select whether enterin...
Page 104
9-8 art: 714371-01f rev . Date: 25-mar -16 apply qc schedule to the months of the year to which this schedule will apply. Options are: all months selected months: check the box next to the months to which this schedule will apply. All months cartridge qc profile the system administrator defines a qc...
Page 105
Art: 714371-01f rev . Date: 25-mar -16 9-9 operator test selection requires the operator to select tests to be reported from a cartridge test panel. Disabled this option facilitates compliance with medicare/medicaid regulations in the usa. Act options (i-stat 1 analyzer only) the user can select bet...
Page 106
9-10 art: 714371-01f rev . Date: 25-mar -16 preference window: for b arcodes option description default comments id barcodes * the user can select any or all of the following as valid barcode formats for both the operator and patient id: • i2 of 5 • code 128 • codabar • code 93 • code 39 • ean 8, ea...
Page 107
Art: 714371-01f rev . Date: 25-mar -16 9-11 * also, tco2 and anion gap, except: 03 tco2 meq/l 04 tco2, anion gap mmol/l 06 anion gap, hco3, be meq/l note: there are no units for ph or for hematocrit when reported as decimal fraction note: see cartridge and test information sheets for act, pt/inr, ct...
Page 108
9-12 art: 714371-01f rev . Date: 25-mar -16.
Page 109: Technical Bulletin
Art: 730009-01b rev. Date: 09-aug-13 technical bulletin i-stat ® reportable range customization on the i-stat ® 1 handheld overview as part of the on-going readi initiative ( responds, enhances, and delivers innovation), abbott point of care (apoc) has released a new custom reportable range feature ...
Page 110
Art: 730009-01b rev. Date: 09-aug-13 2 note: when running controls, if the handheld is customized for user defined reportable ranges and auto pass/fail detection, the handheld will display the control result based upon the customized reportable range setting, but will base the auto pass/fail determi...
Page 111
3 art: 730009-01b rev. Date: 09-aug-13 customizing reportable ranges on the i-stat 1 handheld using cds version 5 1. Click on main g open administration function g customization 2. Type in your password and click ok. The default password is the word istat. Note: abbott point of care inc. Recommends ...
Page 112
Art: 730009-01b rev. Date: 09-aug-13 4 5. Once the preferences window opens, click on the results tab. 6. In the custom reportable ranges section, scroll down to the analyte row which you wish to customize, and click on the corresponding low or high box for that analyte. Type in the customized repor...
Page 113
5 art: 730009-01b rev. Date: 09-aug-13 customizing reportable ranges on the i-stat 1 handheld using i-stat/de 1. Access the customization workspace • rals-plus users: o rals-plus application, pick i-stat from the drop-down menu. O click on device customization. • precisionweb users:: o enter the de ...
Page 114
Art: 730009-01b rev. Date: 09-aug-13 6 5. In the custom reportable ranges section, use the page numbers below the table to find the analyte row which you wish to customize, and click edit at the right end of that row. Type in the customized reportable range value for the low or high reportable range...
Page 115
7 art: 730009-01b rev. Date: 09-aug-13 confirming that the custom reportable ranges have been transferred to the i-stat 1 handheld from cds version 5 or i-stat/de 1. Power on the i-stat 1 handheld and press menu once to get to the administration menu. 2. Press 4 -customization. 3. Press 1-view.. 4. ...
Page 116
Art: 730009-01b rev. Date: 09-aug-13 8 5. Press 2-display ranges. 6. View the displayed reportable ranges. If the analyte customized for reportable ranges does not appear on the first page of the screen, press the gkey to find the page containing the analyte which has the customized display range an...
Page 117: Technical Bulletin
Abbott point of care inc. • abbott park, il 60064 • usa art: 730077-01c rev. Date: 21-mar-14 technical bulletin i-stat ® liquid quality control schedule and lockout customization on the i-stat ® 1 handheld overview as part of the on-going readi initiative ( responds, enhances, and delivers innovatio...
Page 118
Art: 730077-01c rev. Date: 21-mar-14 2 b. Qc scheduling the system administrator associates each qc test profile with at least one of three definable liquid qc schedules. Each schedule can accommodate up to eight (8) qc test profiles. The liquid qc schedule has an administrator-definable “due time” ...
Page 119
3 rev. Date: 21-mar-14 art: 730077-01c caution use of the liquid quality control scheduler and lockout customization features will result in handhelds being unavailable for patient testing when quality control requirements are not met. Minimum software requirements______________________________ the ...
Page 120
Art: 730077-01c rev. Date: 21-mar-14 4 customizing liquid qc schedules on the i-stat 1 handheld using cds version 5 1. Click on main → open administration function → customization 2. Type in your password and click ok. The default password is the word istat. Note: abbott point of care inc. Recommend...
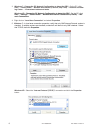
Page 121
5 rev. Date: 21-mar-14 art: 730077-01c 5. Once the preferences window opens, click on the cartridge qc tab. 6. Click on the liquid qc settings at the bottom of the screen. 7. In the control pass/fail determination section, click the radio button for the way in which you will determine the acceptabil...
Page 122
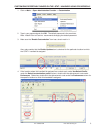
Art: 730077-01c rev. Date: 21-mar-14 6 8. If you want users to enter a comment code when liquid qc results are in-range, out-of-range, or under both situations, check the appropriate box in the control test settings section and then use the drop down menu to select whether entering the comment code ...
Page 123
7 rev. Date: 21-mar-14 art: 730077-01c 10. Select the method in which control lot number information will be entered into the handheld. • scan or enter: allows the user the option of manually entering the liquid qc lot information into the handheld, or scanning it from the barcode on the quality con...
Page 124
Art: 730077-01c rev. Date: 21-mar-14 8 14. The grace period is the period of time, starting from the due time, during which the qc test profile must be completed before the corresponding cartridge set is locked out. Enter the grace period in hours: • up to 23 hours for daily schedules, • up to 167 h...
Page 125
9 rev. Date: 21-mar-14 art: 730077-01c 17. Select the qc cartridge type from the drop-down menu. The qc cartridge is the cartridge type to be tested with specified liquid qc fluids during the qc procedure note 17.1: selecting [none] will cease your ability to proceed through the remaining qc schedul...
Page 126
Art: 730077-01c rev. Date: 21-mar-14 10 22. Once all schedules have been created and defined, click ok and answer yes to the question about changing the preferences. 23. Download the handheld(s) to the cds from a downloader in the location to which the handheld is assigned. This action will upload t...
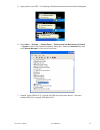
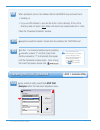
Page 127
11 rev. Date: 21-mar-14 art: 730077-01c customizing liquid quality schedule and lockout features on the i-stat 1 handheld using i-stat/de 1. Access the customization workspace • rals-plus users: o within the rals-plus application, pick i-stat from the drop-down menu. O click on device customization....
Page 128
Art: 730077-01c rev. Date: 21-mar-14 12 4. Once the preferences window opens, click on the cartridge qc tab. 5. Click on liquid qc settings at the top of the screen. 6. In the control pass/fail determination section, click the radio button for the way in which you will determine the acceptability of...
Page 129
13 rev. Date: 21-mar-14 art: 730077-01c note 7.2: the control test settings feature is also customizable through the i-stat 1 handheld keypad. 1. Power on the i-stat 1 handheld and press menu to get to the administration menu 2. Press 4 – customization 3. Press 2 – change 4. Type in your password an...
Page 130
Art: 730077-01c rev. Date: 21-mar-14 14 note 9.1: the apoc fluid lot entry method is also customizable through the i-stat 1 handheld keypad. 1. Power on the i-stat 1 handheld and press menu to get to the administration menu 2. Press 4 – customization 3. Press 2 – change 4. Type in your password and ...
Page 131
15 rev. Date: 21-mar-14 art: 730077-01c 14. The grace period is the period of time, starting from the due time, during which the qc test profile must be completed before the corresponding cartridge set is locked out. Enter the grace period in hours: • up to 23 hours for daily schedules, • up to 167 ...
Page 132
Art: 730077-01c rev. Date: 21-mar-14 16 18. In the fluids section, select up to six (6) types and levels of control fluid that will be required to be run on the handheld during this qc cartridge schedule timeframe and click update. Note 18.1: all i-stat control fluid types and levels are listed in t...
Page 133
17 rev. Date: 21-mar-14 art: 730077-01c new i-stat 1 handheld display screens for the liquid qc schedule and lockout customization features after customizing the i-stat 1 handheld for the new liquid qc schedule and lockout customization features, users may encounter some new handheld display screens...
Page 134
Art: 730077-01c rev. Date: 21-mar-14 18 note 3.1: if this message appears, users can press the listed number on the handheld keypad to display which cartridges are currently inactive. 4. “i-stat qc past due – cartridge testing disabled”: this message will appear if the liquid qc requirements for a s...
Page 135
19 rev. Date: 21-mar-14 art: 730077-01c the user has the following options to choose from on this screen: • 1-unscheduled: selecting this option allows the user to perform a liquid qc run which will not be applied to a customized liquid qc schedule(s). • 2-schedule 1, 3-schedule 2 (if applicable), o...
Page 136
Art: 730077-01c rev. Date: 21-mar-14 20 appendix: liquid control lot abbreviations the table below lists each i-stat control type and corresponding abbreviation used in the cartridge qc fluids drop down menus in the customization workspace. Control name control customization abbreviation i-stat ctni...
Page 137: Technical Bulletin
Abbott point of care inc. • abbott park, il 60064 • usa art: 730078-01c rev. Date: 29-jul-14 technical bulletin i-stat ® liquid quality control pass/fail customization on the i-stat 1 handheld overview as part of the on-going readi initiative ( responds, enhances, and delivers innovations), abbott p...
Page 138
Art: 730078-01c rev. Date: 29-jul-14 2 general notes and considerations 1. The qc auto p/f customization feature is only available on the i-stat 1 handheld, and not the i-stat portable clinical analyzer (i-stat 200 series model). 2. In order to use the qc auto p/f customization feature, users must h...
Page 139
3 rev. Date: 29-jul-14 art: 730078-01c downloading an electronic value assignment sheet (evas) from the apoc website for use with the cds or i-stat/de customization workspaces 1. Navigate to the evas page of the apoc website: www.Abbottpointofcare.Com/evas. 2. Choose the clew from the dropdown menu....
Page 140
Art: 730078-01c rev. Date: 29-jul-14 4 customizing the i-stat 1 handheld for liquid qc p/f using cds version 5 1. Click on main g open administration function g customization. 2. Type in your password and click ok. The default password is the word istat. Note: abbott point of care inc. Recommends ch...
Page 141
5 rev. Date: 29-jul-14 art: 730078-01c 5. If the location where this handheld is assigned has a check mark under use default profile, under the default customization profile: column, double click the alphanumeric code under preferences. Otherwise, double click the alphanumeric code under the prefere...
Page 142
Art: 730078-01c rev. Date: 29-jul-14 6 9. If you want users to enter a comment code when liquid qc results are in-range, out-of-range, or under both situations, check the appropriate box in the control test settings section and then use the drop down menu to select whether entering the comment code ...
Page 143
7 rev. Date: 29-jul-14 art: 730078-01c 11. Select the method in which control lot number information will be entered into the handheld. • scan or enter: allows the user the option of manually entering the liquid qc lot information into the handheld or scanning it from the barcode on the quality cont...
Page 144
Art: 730078-01c rev. Date: 29-jul-14 8 customizing the i-stat 1 handheld for liquid qc p/f using i-stat/de 1. Access the customization workspace • rals-plus users: o within the rals-plus application, pick i-stat from the drop-down menu. O click on device customization. • precisionweb users: o enter ...
Page 145
9 rev. Date: 29-jul-14 art: 730078-01c 8. At the bottom of the default customization profile: column, check the use evas box. 9. If the location where this handheld is assigned has a check mark under uses default, under the default customization profile: column, double click the alphanumeric code un...
Page 146
Art: 730078-01c rev. Date: 29-jul-14 10 12. In the control pass/fail determination section, click the auto via evas radio button. Choosing this option indicates that the handheld will automatically determine whether the liquid qc run passed or failed, based upon qc ranges contained on an electronic ...
Page 147
11 rev. Date: 29-jul-14 art: 730078-01c 14. Select the way in which you would like control results to be displayed. • numeric: liquid qc results are displayed in numeric format. • suppressed: the following symbol “” is displayed next to each liquid qc test name in place of the quantitative (numeric)...
Page 148
Art: 730078-01c rev. Date: 29-jul-14 12 16. Click ok and answer ok to the question about changing the preferences. 17. Download the handheld(s) to the i-stat/de from a downloader in the location to which the handheld is assigned. This action will upload the chosen customization features into the han...
Page 149
13 rev. Date: 29-jul-14 art: 730078-01c confirming that the evas has been transferred to the i-stat 1 handheld from cds version 5 or i-stat/de 1. Power on the i-stat 1 handheld and press menu once to get to the administration menu. 2. Press 4 -customization. 3. Press 1-view. 4. Press 4-qc tests..
Page 150
Art: 730078-01c rev. Date: 29-jul-14 14 5. Press 2-cartridge qc. 6. Press the g key to view the evas name that has been transferred to the handheld..
Page 151
15 rev. Date: 29-jul-14 art: 730078-01c new i-stat 1 handheld display screens for the liquid qc pass/fail customization feature after customizing the i-stat 1 handheld for the new liquid qc auto p/f customization feature, users may encounter some new handheld display screens. Quality tests menu: if ...
Page 152
Art: 730078-01c rev. Date: 29-jul-14 16 if a non-apoc fluid has been selected, the user will then be asked to scan or enter the control lot number. If an apoc fluid has been selected, the user will then be asked to scan the control lot number. Or liquid qc results screens: if the i-stat 1 handheld i...
Page 153
17 rev. Date: 29-jul-14 art: 730078-01c • suppressed control result display with a passed qc run: all analyte results will display a symbol and the overall pass assessment appears at the top of the display screen above the results. • suppressed control result display with a failed qc run: all analyt...
Page 154
Art: 730078-01c rev. Date: 29-jul-14 18.
Page 155: Technical Bulletin
Abbott point of care inc. • 100 and 200 abbott park road • abbott park, il 60064 • usa art: 730211-01c rev. Date: 08-jul-16 technical bulletin i-stat ® positive patient identification (ppid) customization on the i-stat 1 handheld overview as part of the on-going readi initiative ( responds, enhances...
Page 156
Art: 730211-01c rev. Date: 08-jul-16 2 cautions 1. If the lockout override is disabled, a patient test cannot be run unless the entered patient id is in the handheld’s internal patient list. 2. In order to ensure that the patient list stored in the handheld is current, the i-stat 1 handhelds must be...
Page 157
3 rev. Date: 08-jul-16 art: 730211-01c b. Identifier formats the format for the primary and secondary identifiers displayed on the handheld screen is as follows: primary identifier format patient id (e.G. Medical record number or account number) up to 15 characters secondary identifier format last n...
Page 158
Art: 730211-01c rev. Date: 08-jul-16 4 enter year of birth the operator enters the four digits of the patient’s year of birth to confirm the patient id. The date of birth is not displayed on the handheld screen. 2. Not on list action: the system administrator can select the desired behavior when the...
Page 159
5 rev. Date: 08-jul-16 art: 730211-01c customizing the positive patient id feature on the i-stat 1 handheld using i-stat/de 1. Access the customization workspace • rals-plus users: o within the rals-plus application, pick i-stat from the drop-down menu. O click on device customization. • precisionwe...
Page 160
Art: 730211-01c rev. Date: 08-jul-16 6 4. If the location where this handheld is assigned has a check in the uses default column, click the button displaying the alphanumeric code under preferences in the default customization profile: area. Otherwise, click the button displaying the alphanumeric co...
Page 161
7 rev. Date: 08-jul-16 art: 730211-01c 8. Using the drop down menu, select the confirmation method the operator will use to confirm the patient identity: • confirm: the operator confirms the patient id by selecting “continue”. • replicate year of birth: the operator enters the four digits of the pat...
Page 162
Art: 730211-01c rev. Date: 08-jul-16 8 confirming that the patient list has been transferred to the i-stat 1 handheld from i-stat/de 1. Power on the i-stat 1 handheld and press menu once to get to the administration menu. 2. Press 4 -customization. 3. Press 1-view.. 4. Press 2-id entry..
Page 163
9 rev. Date: 08-jul-16 art: 730211-01c 5. Press 2-patient id. 6. Press the g key twice to view the patient list number that has been transferred to the handheld..
Page 165: Technical Bulletin
Technical bulletin i-stat ® abbott point of care inc. • 100 & 200 abbott park road, abbott park, il 60064 • usa art: 730292-01b rev. Date: 13-feb-17 operator competency notification on the i-stat 1 handheld overview as part of the on-going readi initiative ( responds, enhances, and delivers innovati...
Page 166
Art: 730292-01b rev. Date: 13-feb-17 2 customizing operator competency notification on the i-stat 1 handheld using cds version 5.27a and above note: abbott point of care recommends that customers use the current version of central data station. 1. Open the customization workspace. 2. If the location...
Page 167
3 rev. Date: 13-feb-17 art: 730292-01b 3. Once the preferences window opens, click on the instrument tab. 4. In the i-stat reserved data section, type “ce=xxx”, where xxx indicates the number of days (between 1 and 255) in which the operator will be notified of their certification expiration. 5. Cli...
Page 168
Art: 730292-01b rev. Date: 13-feb-17 4 5. Click ok and then answer yes to the question about changing the preferences. 6. Download the handheld(s) to i-stat / de from a downloader in the location to which the handheld is assigned. This action should upload the chosen customization features into the ...
Page 169
Sample collection 10 art: 714372-01m rev. Date: 27-jul-16 10-1 specimen collection overview the specimen used to fill a cartridge must be collected and handled properly to ensure that the results represent the patient’s current status. Specimens should be collected according to the facility’s polici...
Page 170
10-2 art: 714372-01m rev. Date: 27-jul-16 hemolysis avoid hemolysis (bursting of red cells) by • allowing residual alcohol to dry over the puncture site • discarding a sample from a traumatic draw. Hemolysis will cause an increase in potassium results and a decrease in calcium results. For ctni, ck-...
Page 171
Art: 714372-01m rev. Date: 27-jul-16 10-3 i-stat ctni and ck-mb cartridges require the use of either: 1. Heparinized whole blood or plasma samples collected in syringes or evacuated tubes containing lithium or sodium heparin, or 2. Non-heparinized whole blood samples tested within one minute of draw...
Page 172
10-4 art: 714372-01m rev. Date: 27-jul-16 time to test for the most accurate results, test samples immediately after drawing. Samples for lactate must be tested immediately. Samples for ph, pco 2 , po 2 , tco 2 , and ionized calcium should be tested within 10 minutes. Other analytes should be tested...
Page 173
Art: 714372-01m rev. Date: 27-jul-16 10-5 arterial puncture - blood gas, electrolyte, chemistry, and hematocrit tests evacuated tubes evacuated or other blood collection tubes are not recommended for blood gas analysis. Syringes and anticoagulant if the sample can be tested in a cartridge immediatel...
Page 174
10-6 art: 714372-01m rev. Date: 27-jul-16 mix mix blood (whether anticoagulated or not) by rolling between the palms for at least 5 seconds, each in two different directions. Then invert the syringe repeatedly for at least 5 seconds. Discard the first 2 drops of blood. Exposure to air avoid or remov...
Page 175
Art: 714372-01m rev. Date: 27-jul-16 10-7 indwelling line blood gas, electrolyte, chemistry back flush line with a sufficient amount of blood to remove intravenous solutions, heparin or medications that may contaminate the sample. Five to six times the volume of the catheter, connectors and needle i...
Page 176
10-8 art: 714372-01m rev. Date: 27-jul-16 time to test test samples collected in capillary tubes immediately to avoid clotting (especially in neonates whose blood may clot more quickly). Warming area blood flow can be stimulated by warming the puncture site. Follow the facility’s policy and procedur...
Page 177
Art: 714372-01m rev. Date: 27-jul-16 10-9 references 1. Clsi. H3-a4, procedure for the collection of diagnostic blood speci- mens by venipuncture, 4 th ed.; approved guideline, clsi document h3-a4 [isbn 1-56238-350-7]. Clsi, 940 west valley road, suite 1400, wayne, pennsylvania 19087 usa, 1998. 2. C...
Page 179
Procedure for handling cartridges 11 art: 714373-01g rev. Date: 15-aug-16 11-1 preparation for testing select the cartridge select the appropriate cartridge for the test or tests required. While the cartridge is not fragile, it should be handled as follows to avoid difficulty in filling and quality ...
Page 180
11-2 art: 714373-01g rev. Date: 15-aug-16 step action 1 place the cartridge on a flat surface. Note the location of the sample well and fill mark indicator, as identified in the images below. 2 mix the sample thoroughly. A. Invert a blood collection tube at least 10 times. B. Roll a syringe repeated...
Page 181
Art: 714373-01g rev. Date: 15-aug-16 11-3 step action 4 dispense a small amount of sample, ensuring it travels toward the fill mark before applying additional sample. Avoid creating a bubble on the sample well. A. Continue dispensing until the sample reaches the fill mark indicated on the cartridge ...
Page 182
11-4 art: 714373-01g rev. Date: 15-aug-16 step action 5 fold the snap closure over the sample well: a. Keeping your thumb or finger on the outside edge of the closure clasp, press the rounded end of the closure until it snaps into place b. Ensure that the cartridge is completely closed before insert...
Page 183
Art: 714373-01g rev. Date: 15-aug-16 11-5 examples of under-filled cartridges these images display under-filled cartridges. In the images on the left, the sample well is insufficiently filled, and the sample does not reach the fill mark indicator. In the images on the right, the sample well is suffi...
Page 184
11-6 art: 714373-01g rev. Date: 15-aug-16 step action 1 remove cartridge from foil pouch and place the cartridge on a flat surface. 2 prepare lancet device and set aside until needed. 3 clean and prepare the finger to be sampled using a 70% aqueous solution of isopropanol (70% v/v). Allow the finger...
Page 185
Art: 714373-01g rev. Date: 15-aug-16 11-7 inserting and removing the cartridge from the analyzer step action inserting cartridge into analyzer 1 align the cartridge with the contact pads facing up and toward the cartridge port. 2 push the cartridge slowly and smoothly into the cartridge port until i...
Page 186
11-8 art: 714373-01g rev. Date: 15-aug-16 incorrect procedure overview the cartridge is designed to fill and seal correctly. However, the conditions described below may occur, especially during the training period. If the condition is not detected by the operator, the analyzer will detect the condit...
Page 187
Procedure for cartridge testing 12 art: 714374-01m rev. Date: 23-feb-16 12-1 caution the following cautions should be taken to prevent damage to the analyzer and to ensure the safety of the operator and the integrity of results. • never look into the barcode scanner beam or point it toward anyone’s ...
Page 188
12-2 art: 714374-01m rev. Date: 23-feb-16 performing patient analysis 1. Press to turn on the handheld. 2. Press for i-stat cartridge. 3. Follow the handheld prompts. 4. Scan the lot number on the cartridge pouch. • position barcode 3-9 inches from scanner window on the handheld. • press and hold to...
Page 189
Art: 714374-01m rev. Date: 23-feb-16 12-3 results display interpretation of displayed results test results are displayed with numerical concentration values in the units selected for the customization profile. For patient test results, bar graphs depicting the values in relation to reference ranges ...
Page 190
12-4 art: 714374-01m rev. Date: 23-feb-16 flags when the analyzer detects an out-of-range result or an uncharacteristic sensor signal, the condition is indicated by a flag. See table below for flags and symbols used with results. Note: the reportable range flags do not apply when testing is performe...
Page 191
Art: 714374-01m rev. Date: 23-feb-16 12-5 troubleshooting warning message if testing is disabled due to warning message, the condition must be corrected and the analyzer must be turned off and back on again before testing is enabled. Message and quality check code see troubleshooting section. *** in...
Page 193
Art: 714375-01f rev. Date: 31-jan-12 13 procedures for glucose test strip testing upon installation of the april 2012 software update, the glucose test strip port functionality will be permanently disabled on all i-stat 1 handhelds. Note: the remaining technical information regarding the glucose str...
Page 194
13-2 art: 714375-01f rev. Date: 31-jan-12.
Page 195: Quality Control
Quality control 14 art: 714376-01p rev. Date: 27-jan-17 14-1 overview this section describes the steps to be taken to verify the performance of the analyzer and cartridges. The rationale for the i-stat cartridge and analyzer quality regimen is described in the theory section of this manual. Customiz...
Page 196
14-2 art: 714376-01p rev. Date: 27-jan-17 i-stat analyzers contain a thermal control subsystem consisting of two thermal probes with thermistors and heating contact wires. When measurements are performed at a controlled temperature, the thermal probes in the analyzer contact the metalized area under...
Page 197
Art: 714376-01p rev. Date: 27-jan-17 14-3 controls for blood gas/electrolyte/metabolite cartridges control solutions aqueous assayed control fluids are available for verifying the integrity of newly received cartridges. I-stat level 1, 2 and 3 controls are formulated at three clinically relevant lev...
Page 198
14-4 art: 714376-01p rev. Date: 27-jan-17 procedure step action 1 access the i-stat cartridge control option under quality tests in the administration menu. Enter the required information. The analyzer allows 15 minutes (or the customized timeout) to insert the cartridge after the last data entry. 2...
Page 199
Art: 714376-01p rev. Date: 27-jan-17 14-5 the ranges displayed represent the maximum deviation expected when controls and cartridges are performing properly. Should results outside the ranges be obtained, refer to the troubleshooting section that follows the procedure for testing controls. Target va...
Page 200
14-6 art: 714376-01p rev. Date: 27-jan-17 controls for blood gas/electrolyte/metabolite cartridges (i-stat tricontrols) control solutions aqueous-based control fluids are available for verifying the integrity of newly received cartridges. I-stat tricontrols level 1, 2 and 3 are formulated at three c...
Page 201
Art: 714376-01p rev. Date: 27-jan-17 14-7 before use i-stat tricontrols solutions require different temperature stabilization times depending on whether or not po 2 is to be measured. If po 2 is to be measured, equilibrate the ampule to room temperature for 4 hours prior to use. If po 2 is not being...
Page 202
14-8 art: 714376-01p rev. Date: 27-jan-17 target values target values (determined by testing multiple ampules of each level using multiple lots of cartridges and i-stat handhelds that have passed the electronic simulator test) are printed on a value assignment sheet posted on the apoc website at www...
Page 203
Art: 714376-01p rev. Date: 27-jan-17 14-9 the acceptance limits which have been established for these control solutions are wider than analogous limits established for the current i-stat control and calibration verification solutions, reflecting the precision effect highlighted above. The situation ...
Page 204
14-10 art: 714376-01p rev. Date: 27-jan-17 warnings and precautions handle this product using the same safety precautions used when handling any potentially infectious material. The human plasma used in the preparation of this product has been tested by fda approved test methods and found negative/n...
Page 205
Art: 714376-01p rev. Date: 27-jan-17 14-11 controls for pt/inr cartridges intended use the i-stat ® pt control level 1 (normal) and pt control level 2 (abnormal) are used to verify the integrity of newly received pt/inr cartridges. Contents each level of control is packaged as a box of 5 vials of ly...
Page 206
14-12 art: 714376-01p rev. Date: 27-jan-17 control target values and expected ranges target values (determined by testing multiple vials of each level using multiple lots of i-stat cartridges with analyzers that have passed the electronic simulator test) are printed on a value assignment sheet poste...
Page 207
Art: 714376-01p rev. Date: 27-jan-17 14-13 control target values and ranges see value assignment sheets posted on the apoc website at www.Pointofcare.Abbott. The value assignment sheet displays target values and ranges expected when cartridges, controls, and equipment are performing properly. Always...
Page 208
14-14 art: 714376-01p rev. Date: 27-jan-17 performing electronic simulator test procedure for internal electronic simulator the internal electronic simulator test cycle is automatically activated when a cartridge is inserted after the customized interval is reached. If the analyzer passes the simula...
Page 209
Art: 714376-01p rev. Date: 27-jan-17 14-15 caution the analyzer will continue to initialize test cycles when the analyzer is customized to warn, but not block testing when a scheduled external electronic simulator test is missed, when a fail result for the external electronic simulator test is ignor...
Page 210
14-16 art: 714376-01p rev. Date: 27-jan-17 verify the thermal probe check for the i-stat 1 analyzer as follows: • external electronic simulator used routinely and results transmitted to a central data station version 5: on the cds, click on data viewer, then on simulator. Look under the probe delta ...
Page 211
Art: 714376-01p rev. Date: 27-jan-17 14-17 performing control test on cartridge procedure for testing controls initiating control tests from the quality test menu allows results to be stored in separate categories for the purpose of documentation and review. • do not insert cartridge to start test. ...
Page 212
14-18 art: 714376-01p rev. Date: 27-jan-17 this page intentionally left blank.
Page 213: I-St
Art: 714376-01p rev. Date: 27-jan-17 14-19 i-st a t system incoming cartridge qc log cartridge t ype:____________ lot no.:____________ rec’d date:____________ exp. Date:____________ quant:______________ t emp. Strip:______________ contr ol name: ____________________________ level: __________________...
Page 214
14-20 art: 714376-01p rev. Date: 27-jan-17 this page intentionally left blank.
Page 215: I-St
Art: 714376-01p rev. Date: 27-jan-17 14-21 i-st a t system qc log: expiration date and storage conditions refrigera ted 2 to 8° c (35 to 46° f) room tempera ture 18 to 30° c (64 to 86° f) da te loca tion car tridge type lot # qty exp . Da te temp qty exp . Da te temp actions insp ..
Page 216
14-22 art: 714376-01p rev. Date: 27-jan-17 this page intentionally left blank.
Page 217: I-St
Art: 714376-01p rev. Date: 27-jan-17 14-23 i-st a t cartridge quality contr ol action log da te time control level control lot car tridge lot problem corrective action opera tor.
Page 218
14-24 art: 714376-01p rev. Date: 27-jan-17 this page intentionally left blank.
Page 219: I-St
Art: 714376-01p rev. Date: 27-jan-17 14-25 i-st a t electr onic simulator log for analyzer serial number:________________ y ear:_________ da te time pass fail simula tor id opera tor time pass fail simula tor id opera tor time pass fail simula tor id opera tor.
Page 220
14-26 art: 714376-01p rev. Date: 27-jan-17 this page intentionally left blank.
Page 221: I-St
Art: 714376-01p rev. Date: 27-jan-17 14-27 i-st a t electr onic simulator action log da te time anal yzer failure code or letter simula tor id action opera tor.
Page 222
14-28 art: 714376-01p rev. Date: 27-jan-17 this page intentionally left blank.
Page 223: I-St
Art: 714376-01p rev. Date: 27-jan-17 14-29 i-st a t analyzer thermal pr obe check year: ________ analyzer serial no. : ___________________________ da te simula tor serial no. Thermal probe del ta resul t acceptable range : -0.1 to +0.1 comments opera tor analyzer serial no. : _______________________...
Page 224
14-30 art: 714376-01p rev. Date: 27-jan-17 this page intentionally left blank.
Page 225
Calibration verification 15 art: 714377-01o rev. Date: 27-jan-17 15-1 calibration verification for blood gas/electrolyte/metabolite cartridges purpose calibration verification is a procedure intended to verify the accuracy of results over the entire measurement range of a test. The performance of th...
Page 226
15-2 art: 714377-01o rev. Date: 27-jan-17 before use i-stat calibration verification solutions require different temperature stabilization times depending on whether or not oxygen is to be measured. If oxygen is to be measured, equilibrate the ampule to room (ambient) temperature for 4 hours. If not...
Page 227
Art: 714377-01o rev. Date: 27-jan-17 15-3 acceptable criteria target values (determined by testing multiple ampules of each level using multiple lots of i-stat cartridges with analyzers that have passed the electronic simulator test) are printed on a value assignment sheet posted on the apoc website...
Page 228
15-4 art: 714377-01o rev. Date: 27-jan-17 procedure step action 1 immediately before use, shake the ampule vigorously for 5 to 10 seconds to equilibrate the liquid and gas phases. To shake, hold the ampule at the tip and bottom with forefinger and thumb to minimize increasing the temperature of the ...
Page 229
Art: 714377-01o rev. Date: 27-jan-17 15-5 calibration verification for blood gas/ electrolyte/ metabolite cartridges (i-stat tricontrols) purpose calibration verification is a procedure intended to verify the accuracy of results over the entire measurement range of a test. The performance of this pr...
Page 230
15-6 art: 714377-01o rev. Date: 27-jan-17 storage refrigerated storage at 2-8 ºc (35-46 ºf) should be maintained until the printed expiration date on the box and ampule labels. Tricontrols solutions may also be maintained at room temperature (18-30 ºc; 64-86 ºf) for up to 5 days. Do not use tricontr...
Page 231
Art: 714377-01o rev. Date: 27-jan-17 15-7 transfer with syringe plain syringes (fresh 1 ml or 3 ml sterile syringe with 16 – 20 gauge needles) are recommended to transfer aqueous calibration verification solutions from the ampule to the cartridge. When using a syringe, slowly draw approximately 1 ml...
Page 232
15-8 art: 714377-01o rev. Date: 27-jan-17 interpretation of results the i-stat hematocrit method using blood anticoagulated with lithium heparin is calibrated to give results equivalent to the reference microhematocrit method using blood anticoagulated with k 3 edta. Since the blood used for the mic...
Page 233
Art: 714377-01o rev. Date: 27-jan-17 15-9 calibration verification for i-stat ctni, bnp, and ck-mb cartridges intended use: the i-stat ctni, bnp, and ck-mb calibration verification sets are intended for use as an assayed plasma material to verify the greater portion of the reportable range for i-sta...
Page 234
15-10 art: 714377-01o rev. Date: 27-jan-17 target values are specific to the i-stat system. Results may differ if used with other methods. If a result for a level is outside the range published in the value assignment sheet, two additional cartridge runs should be performed on this level and the thr...
Page 235
Proficiency or external quality control testing 16 art: 714378-01e rev. Date: 31-jan-12 16-1 purpose samples from external quality control providers can be used to assess consistency of results for a particular method or system across testing sites. Due to matrix effects and additives, these samples...
Page 236
16-2 art: 714378-01e rev. Date: 31-jan-12 troubleshooting the i-stat system is designed to measure fresh whole blood samples. Matrix effects and interfering substances can be expected when measuring non-whole blood samples. The following points should be considered when selecting and testing externa...
Page 237
Routine care of the analyzer and downloader 17 art: 714379-01g rev. Date: 07-mar-17 17-1 drying a wet analyzer or downloader if the analyzer is placed on a wet surface or if any liquid is spilled onto it, dry the analyzer immediately. If liquid enters the following compartments, the analyzer may be ...
Page 238
17-2 art: 714379-01g rev. Date: 07-mar-17 procedure step action 1 prepare a 1:10 solution of household bleach by mixing one part of bleach with nine parts of tap water. This solution will maintain its germicidal action for a week. 2 soak a few gauze pads in the bleach solution. Before use, squeeze t...
Page 239
Art: 714379-01g rev. Date: 07-mar-17 17-3 removing and replacing the rechargeable battery wait until any test in progress is completed, and turn off the analyzer before replacing the battery or the most recent set of results may be lost. Stored results will not be lost when replacing the batteries. ...
Page 240
17-4 art: 714379-01g rev. Date: 07-mar-17.
Page 241: Updating The Software
Updating the software 18 art: 714380-01j rev. Date: 04-sep-13 18-1 for instructions on updating your i-stat ® 1 handheld, please refer to the following technical bulletins directly following section 18 in your i-stat 1 system manual: 1. Instructions for updating i-stat 1 handheld software using www....
Page 243: I-Stat
Art: 731335-01b rev. Date 13-aug-13 i-stat ® technical bulletin instructions for updating i-stat ® 1 handheld software using www.Abbottpointofcare.Com overview this technical bulletin has been created specifically to guide you through the process of updating the software on your i-stat ® 1 handheld(...
Page 244
Optional steps once the first i-stat 1 handheld has been updated using the jammlite utility, additional i-stat 1 handhelds may be updated the same way or by using the handheld-to-handheld method. Additionally, if using central data station (cds), the new software must be added to the cds customizati...
Page 245
Art: 731335-01b rev. Date 13-aug-13 3 follow these steps to update with the jammlite utility before starting the process make sure all the required equipment is available: • computer with: • windows 2000, windows xp or windows 7 • a 9-pin serial port or usb port (if using a drc-300) • access to: htt...
Page 246
Art: 731335-01b rev. Date 13-aug-13 4 ensure your i-stat 1 handheld has enough battery power (7.5 volts or higher). To do this: • press the on/off ( ) key • press the menu key • press 1 for analyzer status • confirm the battery voltage rechargeable batteries disposable batteries if you have disposab...
Page 247
Art: 731335-01b rev. Date 13-aug-13 5 connect the power supply: • to the i-stat 1 serial downloader or serial downloader/recharger, and • to a wall outlet or power strip when power is supplied to the serial downloader, a green light will illuminate. When power is supplied to the serial downloader/re...
Page 248
Art: 731335-01b rev. Date 13-aug-13 6 navigate to saved file location. Double click the software file “suxxxxxx.Exe”. When prompted, click on the software file link (suxxxxxx.Exe) and save file to: • desktop, or • if you use cds version 5, save the file to the c:\bins directory. If the c:\bins direc...
Page 249
Art: 731335-01b rev. Date 13-aug-13 7 update 4 4 x instrument jammlite 4.3 jamsxxxx.Bin exit axx.Clw port ip address application clew i-stat 300 analyzer com1 update 4 4 x instrument jammlite 4.3 jamsxxxx.Bin exit axx.Clw port ip address application clew i-stat 300 analyzer com1 if no ports are disp...
Page 250
Art: 731335-01b rev. Date 13-aug-13 8 follow the onscreen instructions. If you do not see the screen shown on the left— —acknowledge the error message(s) and click ok. Return to step when the update is in progress, the following screen will appear: do not move the handheld until the success screen i...
Page 251
Art: 731335-01b rev. Date 13-aug-13 9 run the electronic simulator in the handheld. When the simulator finishes, pass should be displayed. If pass is not displayed, re-run the electronic simulator. If the repeated electronic simulator attempt fails, please contact your support services representativ...
Page 252
Before starting the process make sure all the required equipment is available: • recently updated i-stat 1 handheld (referred to in this section as the sending handheld) charged to 7.5 volts or higher* • the handheld unit to be updated (referred to in this section as the receiving handheld) charged ...
Page 253: 1’’
Art: 731335-01b rev. Date 13-aug-13 11 1 1’’ 1 1’’ if that did not work, a password is needed. Enter the password defined by your facility and press ent. Place sending and receiving handhelds on a flat surface with infrared (ir) windows aligned, approximately 1 foot apart. Turn on the sending handhe...
Page 254
Art: 731335-01b rev. Date 13-aug-13 12 in the utility menu: • press 1-send software • press 1-jamsxxx/axx note: the “numbers” have been replaced with x’s in the example above and will change with each software update. Make sure the receiving handheld’s power is off. When the sending handheld display...
Page 255
Art: 731335-01b rev. Date 13-aug-13 13 when the update is in progress, the sending handheld will display sending along with a bar indicating that the software is being sent. The receiving handheld will have 1’s and 0’s streaming across the screen signifying that it is receiving the software. Do not ...
Page 256
For additional information on running the electronic simulator, please see: • section 14 of the i-stat 1 system manual, or • the introduction and start-up section of the i-stat system manual for waived tests if pass is not displayed, re-run the electronic simulator. If the repeated electronic simula...
Page 257
Art: 731335-01b rev. Date 13-aug-13 15 before starting the process, choose the appropriate cds update path: are you attempting to update cds on the pc that was used to update the i-stat 1 handhelds with jammlite? If “yes,” continue with step if “no,” (cds is not installed on the pc that was used wit...
Page 258
Art: 731335-01b rev. Date 13-aug-13 16 if you are unable to open cds customization, please contact your support services representative and indicate that your cds customization password is unknown. Under the “default customization profile:” column, double click on the “i-stat analyzer clew:” button....
Page 259
Art: 731335-01b rev. Date 13-aug-13 17 location enable updates update clew philips bam clew preferences statnotes location-based customization profiles: a_1 a16 h16 default0 chart0 use default profile i-stat analyzer clew x ok cancel i-stat analyzer a16 h16 philips bam clew i-stat analyzer philips b...
Page 260: Congratulations.
Default customization profile: language: english unit set: unitset00 philips analyzer clew: hxx i-stat 1 software: jamsxxxx.Bin preferences: default0 statnotes: use operator list 09209atj i-stat analyzer clew: axx art: 731335-01b rev. Date 13-aug-13 18 underneath the “default customization profile:”...
Page 261
Art: 731335-01b rev. Date 13-aug-13 19 if cds is not installed on the pc that was used with the jammlite utility: • go to the pc where cds is installed • go to pages 5–7 of the jammlite utility section and complete steps through • when complete, proceed to the cds customization section pages (16–19)...
Page 263: Technical Bulletin
Art: 731336-01b rev. Date: 13-aug-13 technical bulletin i-stat ® network options for updating the i-stat ® 1 handheld using www.Abbottpointofcare.Com overview this technical bulletin has been created specifically to guide you through the process of updat- ing the software on the i-stat ® 1 handheld(...
Page 264
Art: 731336-01b rev. Date: 13-aug-13 2 1. Update procedure using a network downloader and the jammlite process with tcp/ip 1.1: before starting the process, make sure all the required equipment / information is available. • computer with: o windows 2000, xp, or windows 7 o access to http://abbottpoi...
Page 265
3 art: 731336-01b rev. Date: 13-aug-13 1.9: in the jammlite utility, select the i-stat 300 analyzer within the instrument dropdown menu. 1.10: select tcp/ip within the port dropdown menu. 1.11: type the ip address of the network downloader being used for the software update in the ip address box. No...
Page 266
Art: 731336-01b rev. Date: 13-aug-13 4 1.14: when the update is in progress, the following screen will appear : the handheld will have 1’s and 0’s streaming across the screen signifying that it is receiving the software. Do not move the handheld until the success screen is displayed. 1.15: run the e...
Page 267
5 art: 731336-01b rev. Date: 13-aug-13 2. Updating the i-stat 1 handheld using the cds version 5 customization workspace with a serial or network downloader or downloader / recharger, or drc-300 2.1: before starting the process, make sure all the required equipment / information is available: • comp...
Page 268
Art: 731336-01b rev. Date: 13-aug-13 6 a password box will then appear. • type in the password and press enter. The default password is istat. Note: abbott point of care inc. Recommends changing the default password. Note: if you are unable to open cds customization, please contact apoc technical su...
Page 269
7 art: 731336-01b rev. Date: 13-aug-13 • if “use default profile” is not checked beside any location-based customization profile, double click the box under the “i-stat analyzer clew” column. • click the new version of clew, and then click ok. Click yes for the confirmation message. • underneath the...
Page 270
Art: 731336-01b rev. Date: 13-aug-13 8 • the handheld will then display 1’s and 0’s streaming across the screen signifying that it is receiving the software. Once the 1’s and 0’s disappear, the handheld display will again go blank for approximately 5-10 seconds. • a waiting to send message following...
Page 271
9 art: 731336-01b rev. Date: 13-aug-13 3. Updating the i-stat 1 handheld using a serial downloader or serially connected drc-300 and the jammlite process for accounts with rals-plus and i-stat/de 3.1: before starting the process, make sure all the necessary required equipment is available. • compute...
Page 272
Art: 731336-01b rev. Date: 13-aug-13 10 • record “ host” and “id” entry. • uncheck the “ enabled” box. Click ok. • if not enabled, minimize the window and proceed to the next step. 3.5: navigate to www.Abbottpointofcare.Com/softwareupdate. • select language from the drop-down menu. • indicate the da...
Page 273
11 art: 731336-01b rev. Date: 13-aug-13 3.7: in the jammlite utility, select the i-stat 300 analyzer within the instrument drop- down menu. 3.8: make sure there is at least one port number listed under the port drop-down list. Note: if the port drop-down list says “none.” make certain the connection...
Page 274
Art: 731336-01b rev. Date: 13-aug-13 12 3.12: when the update is in progress, the following screen will appear: the handheld will have 1’s and 0’s streaming across the screen signifying that it is receiving the software. Do not move the handheld until the success screen is displayed. 3.13: run the e...
Page 275
13 art: 731336-01b rev. Date: 13-aug-13 3.15: update the clew version in the customization workspace. • transfer the files. O access the main customization workspace page. O click update i-stat/de → upload update file. O browse to desktop, click on and click upload. (note: the xxx is the clew versio...
Page 276
Art: 731336-01b rev. Date: 13-aug-13 14 • check the box next to the new version of clew and click ok. Answer ok to the question that appears. • if “uses default” is not checked beside any location-based customization profile, click the box under the “ i-stat analyzer clew” column. • click the new ve...
Page 277
15 art: 731336-01b rev. Date: 13-aug-13 4. Updating the i-stat 1 handheld using the i-stat/de customization workspace and a network downloader, downloader/recharger, or drc-300 4.1: before starting the process, make sure all the required equipment / information is available. • computer which can acc...
Page 278
Art: 731336-01b rev. Date: 13-aug-13 16 • precisionweb users: o double click on the desktop shortcut or internet explorer favorites for i-stat customization. 4.5: update the clew and jams versions in the customization workspace. • under the “default customization profile:” column, click on the “ i-s...
Page 279
17 art: 731336-01b rev. Date: 13-aug-13 • click the new version of clew, and then click ok. Answer ok to the question that appears. • under the “default customization profile:” column, click on the i-stat 1 software drop- down list. Select the jams version that matches the product update and click o...
Page 280
Art: 731336-01b rev. Date: 13-aug-13 18 • place the handheld in the downloader or downloader / recharger. Do not move the handheld until step 4.8. A communication in progress message will appear on the screen. After this disappears, the handheld display will stay blank for approximately 5-10 seconds...
Page 281: Troubleshooting The Analyzer
Troubleshooting the analyzer 19 rev. Date: 11-mar-14 art: 714381-01j 19-1 introduction when the analyzer detects a potential or real problem before the test cycle is initiated or at any time during the test cycle, a quality check code number, the type of problem and the next step to be taken will be...
Page 282
19-2 art: 714381-01j rev. Date: 11-mar-14 startup messages overview whenever the analyzer is turned on using the on/off key, the analyzer performs self-checks. If a condition that should be corrected in the near future, but that will not affect results is detected, a warning is displayed. The operat...
Page 283
Rev. Date: 11-mar-14 art: 714381-01j 19-3 test cycle messages and quality check codes overview if a problem is detected during a testing cycle, the cycle will be stopped and a message will identify the problem and indicate the next step to be taken. If the problem causes testing to be disabled, the ...
Page 284
19-4 art: 714381-01j rev. Date: 11-mar-14 error in cartridge or fluid movement the following conditions usually indicate an error condition relating in some way to the cartridge or fluid movement within a cartridge. These conditions can be operator or sample related. In most cases a new cartridge mu...
Page 285
Rev. Date: 11-mar-14 art: 714381-01j 19-5 error in cartridge or fluid movement (continued) message on display cause action insufficient sample use another cartridge this is most likely due to insufficient sample in the sample well of the cartridge, but can also be caused by bubbles in the sample. Tr...
Page 286
19-6 art: 714381-01j rev. Date: 11-mar-14 no display symptom possible cause action the display screen remains blank, either after a cartridge has been properly inserted or after the on/off key has been pressed. Batteries dead. Keypad not responding. Internal start switch broken. Change or recharge b...
Page 287: Technical Bulletin
Abbott point of care inc. • 100 & 200 abbott park road • abbott park, il 60064 • usa art: 714260-01r rev. Date: 30-mar-17 technical bulletin i-stat ® analyzer coded messages from the time it powers up until the time it powers down, the i-stat ® analyzer performs numerous quality checks. The failure ...
Page 288
Art: 714260-01r rev. Date: 30-mar-17 2 code number cause/action message on display explanation 2 temperature out of range / check status page the analyzer is recording a temperature outside its operating range. Move the analyzer to an area within the operating temperature of the test being performed...
Page 289
3 rev. Date: 30-mar-17 art: 714260-01r code number cause/action message on display explanation 22, 25 cartridge error / use another cartridge these codes occur only for coagulation cartridges if the mixing of the sample and reagent is compromised. This can be caused by an insufficient or clotted sam...
Page 290
Art: 714260-01r rev. Date: 30-mar-17 4 code number cause/action message on display explanation 21 cartridge preburst / use another cartridge this code indicates that the analyzer detected fluid on the sensors before it should have. Possible causes: mishandling of cartridges (putting pressure in the ...
Page 291
5 rev. Date: 30-mar-17 art: 714260-01r the following conditions are related to electronic or mechanical failures in the analyzer. Code number cause/action message on display explanation 50 analyzer error / use electronic simulator the motor has moved too far. Running a simulator may not detect this ...
Page 292
Art: 714260-01r rev. Date: 30-mar-17 6 code number cause/action message on display explanation 23, 53, 55-57, 63, 65-68, 72-74, 82, 83-85, 86, 89-94, 96, 97 analyzer error / see manual these are mechanical or electronic failures from which the analyzer may not be able to recover. Code 23 may be caus...
Page 293
7 rev. Date: 30-mar-17 art: 714260-01r code number cause/action message on display explanation 69 cartridge type not recognized / use another cartridge this code could be due to use of a cartridge type that is not compatible with the version of software in the analyzer, or the use of expired cartrid...
Page 294
Art: 714260-01r rev. Date: 30-mar-17 8 codes in the range of 120 to 138 and 140 to 151 indicate a failure during an immuno cartridge cycle. In most cases, the cartridge is spent and another cartridge must be used. Code number cause/action message on display explanation 120-122, 124, 125, 133, 144, 1...
Page 295
9 rev. Date: 30-mar-17 art: 714260-01r code number cause/action message on display explanation 141 test canceled by operator this code will be displayed if the cartridge barcode is not scanned within 60 seconds of cartridge insertion. The correct barcode to scan is the barcode on the cartridge porti...
Page 296
Art: 714260-01r rev. Date: 30-mar-17 10 the following conditions are related to the electronic simulator code explanation how to respond numerical code see under analyzer coded messages. See under analyzer coded messages. L potentiometric channel out of limits. Can occur if moisture collects on the ...
Page 297: Theory
Theory 20 art: 714382-01d rev. Date: 01-apr-14 20-1 analyzer functions introduction the i-stat 1 analyzer is a microprocessor-controlled electromechanical instrument designed to: • identifythecartridgetype. • controltheflowoffluidswithinthecartridges. • mixsampleandreagent(whereapplicable). • apply ...
Page 298
20-2 art: 714382-01d rev. Date: 01-apr-14 • amultiplexedpotentiometricsignalline • amultiplexedamperometricsignalline • anacfluidconductivitysignal • a digital identification code to identify the type of cartridge being inserted into the analyzer mechanical system asingledcgearmotordrivesmechanicals...
Page 299
Rev. Date: 01-apr-14 art: 714382-01d 20-3 sensors the general term “sensor” is used to refer to the three types of electrodes incorporated into the cartridges: • potentiometric • amperometric • conductometric sensors are thin film electrodes microfabricated onto silicon chips. Sensing functionalityi...
Page 300
20-4 art: 714382-01d rev. Date: 01-apr-14 activity versus concentration ion-selectiveelectrodesmeasureactivityratherthanconcentration.Activity(a) isrelatedtoconcentration(c)throughtheactivitycoefficient( γ):a=γc. While ion activities, which reflect free rather than total ion concentrations, are the ...
Page 301
Rev. Date: 01-apr-14 art: 714382-01d 20-5 determination of cell concentration cpb eachtimeacartridgecontainingahematocritsensorisused,theoperatorhas theoptionofselecting,inadditiontothesampletype,thecpbcompensation algorithm for samples with abnormally low protein levels. The cpb option is specifica...
Page 302
20-6 art: 714382-01d rev. Date: 01-apr-14 forexample: • uncompensatedhct=21%pcv • 21%pcv=0.50of42% • inferredtotalprotein=7.0g/dlx0.50=3.5g/dl • 21%pcv+3g/dl=24%pcv(cpb) limitations of the cpb algorithm thecpbalgorithmisbaseduponaseriesofinferences: • the algorithm models initial pre-pump values for...
Page 303
Rev. Date: 01-apr-14 art: 714382-01d 20-7 determination of coagulation endpoints act and pt/inr in coagulation tests, the result that is reported is the time required for the process of coagulation to occur. To determine this time, there must be a detectable change in a sample parameter correlated t...
Page 304
20-8 art: 714382-01d rev. Date: 01-apr-14 the use of unit-use cartridges frees the i-stat system from these skilled maintenance and calibration procedures. It also allows for the design of a quality control system which automatically monitors those aspects of the measurementprocesswhicharethemostlik...
Page 305
Rev. Date: 01-apr-14 art: 714382-01d 20-9 comparisonofthisregimentolaboratoryqualitycontrolprocedurescanseem confusing because it does not employ liquid control solutions. However, the principle is the same in that the traditional intermittent quality control measurementsareappliedtothepersistentpar...
Page 306
20-10 art: 714382-01d rev. Date: 01-apr-14 • puttingintoomuchsample • puttingintoolittlesample • rerunningthesamecartridge • introducinganairsegmentintothefluidsegment,etc.The analyzerwillflagtheseconditionsandnotdeliveraresult. 3) the design of some unit-use point-of-care devices can allow an entir...
Page 307
Rev. Date: 01-apr-14 art: 714382-01d 20-11 handheld analyzer verification when verified motorizedmechanicalsystem • verifyelectricalcontactismadewithsensorsoncartridge • verifyabilitytoproperlymovecalibrationfluid,if applicable • verifyabilitytoproperlymovesample everycartridgeuse everycartridgeuse ...
Page 308
20-12 art: 714382-01d rev. Date: 01-apr-14 validating the performance of the i-stat system untilrecently,regulationsandlaboratoryaccreditationstandardsspecifiedthe use of traditional quality control regimens, including the daily use of liquid “control”materials. As new technologies such as the i-sta...
Page 309
Rev. Date: 01-apr-14 art: 714382-01d 20-13 the system quantitatively confirms the accuracy of the mixing step by monitoring the key parameters of mix uniformity, magnitude and timing. Thesequalitytestsareperformedoneachcoagulationcartridge. I-stat’s microfabrication production processes are inherent...
Page 311
Downloader programming and wiring 21 art: 714383-01f rev. Date: 02-sep-08 21-1 p rogramming the n etwork d ownloaders this section includes procedures to configure the network downloaders to transmit data between the i-stat 1 analyzers and a data manager as well as from other peripheral devices to a...
Page 312
21-2 art: 714383-01f rev. Date: 02-sep-08 2. While holding down the x key on the pc keyboard, apply power to the downloader. When the following screen is displayed release the x key: 3. Press the enter key immediately to enter the setup mode: configure server parameters each network downloader requi...
Page 313
Art: 714383-01f rev. Date: 02-sep-08 21-3 1. Determine the following site specific information for this downloader: • ip address (example: 10.10.12.142 used below) • gateway address (example: 10.10.12.1 used below) • netmask (example: 8 for 255.255.255.0 used below) 2. At the your choice? Prompt, se...
Page 314
21-4 art: 714383-01f rev. Date: 02-sep-08 configure for i-stat 1 data transfer via ir port channel 2 provides network access for the i-stat 1 analyzer data transmissions to a data manager. This section describes how to set up parameters for channel 2. 1. Determine the following information: • the ip...
Page 315
Art: 714383-01f rev. Date: 02-sep-08 21-5 2. Save the settings by selecting 9 (save and exit) at the your choice ? Prompt. 3. Remove power and connect the downloader in its intended location. Troubleshooting if a wrong number is entered, which cannot be corrected, press the enter key until the sessi...
Page 316
21-6 art: 714383-01f rev. Date: 02-sep-08 wiring the downloaders overview this section includes diagrams to make a connection between the downloaders and the data manager and to connect a printer to the downloaders. Connecting the network downloader option 1: the following diagram shows how to conne...
Page 317
Art: 714383-01f rev. Date: 02-sep-08 21-7 option 2: the following diagram shows how to connect the portable printer to the network downloader for power and communication. Parts required are: • printer interface cable • printer ac adapter or printer power cable power in power out rj12 (printer interf...
Page 318
21-8 art: 714383-01f rev. Date: 02-sep-08 connecting the serial port downloader option 1: the following option is for downloading/uploading only and can be used when there is no power outlet available for the downloader or downloader/recharger. In this particular configuration, both recharging led l...
Page 319
Art: 714383-01f rev. Date: 02-sep-08 21-9 option 2: the following diagram shows how to connect a serial downloader to the data manager, and to connect the portable printer to the downloader for communication. Parts required are: • db9-db9 null modem cable • printer interface cable • printer ac adapt...
Page 320
21-10 art: 714383-01f rev. Date: 02-sep-08 option 3: the following diagram shows how to connect a serial downloader to the data manager, and to connect the portable printer to the downloader for power and communication. The printer can also be powered by its own ac adpater. Parts required are: • db9...
Page 321
Rev. Date: 16-apr-15 art: 714384-01f 22-1 central data station 5 22 abbott point of care inc. License agreement and warranty for central data station program eula for new users of cds software the license and warranty information in the end user license agreement (eula) will be in effect. Warranty a...
Page 322
22-2 art: 714384-01f rev. Date: 16-apr-15.
Page 323
Rev. Date: 16-apr-15 art: 714384-01f 22-3 installation of the central data station hardware the pc on which the cds software resides must meet specifications provided by abbott point of care inc. And should be installed following the pc manufacturer’s directions. Install the printer if applicable. S...
Page 324
22-4 art: 714384-01f rev. Date: 16-apr-15.
Page 325
Rev. Date: 16-apr-15 art: 714384-01f 22-5 general procedures and conventions overview the cds software follows typical microsoft windows conventions and procedures. The illustrations below are used to point out the use of the menu bar, toolbars, tabs and buttons. Selecting menu options clickingan...
Page 326
22-6 art: 714384-01f rev. Date: 16-apr-15 selecting functions in a window refreshing/ updating the data in a window the refresh toolbar button refreshes the data content in the active window with the most recent data available. The refresh function is also available under the window option on the me...
Page 327
Rev. Date: 16-apr-15 art: 714384-01f 22-7 sorting data in a window inmostcases,whendataispresentedinatable,clickingacolumnheaderwill sort the display based on the data in that column. In the data viewers, repeating values, such as a patient id, will be sorted in descending date/time order. Clickingt...
Page 328
22-8 art: 714384-01f rev. Date: 16-apr-15.
Page 329
Rev. Date: 16-apr-15 art: 714384-01f 22-9 customization of the central data station overview the customization options are: site information institution name and technical support phone number serial ports enables/disables serial communications and allows individual ports to be selected and configur...
Page 330
22-10 art: 714384-01f rev. Date: 16-apr-15 network clickthe enable network communicationsboxtocheckitandenablenetwork communications. Thedefaulttcpserviceportassignmentsarelistedinthenetworktabpage.If notusingthedefaultports,clicktheportandtypeinthenewassignment.Port numbers must be unique and in th...
Page 331
Rev. Date: 16-apr-15 art: 714384-01f 22-11 interface the appropriate interface protocol and the types of records to be sent to another data management system are selected in the interface tab page. This configuration will typically be done by the interface provider. Select the desired primary interf...
Page 332
22-12 art: 714384-01f rev. Date: 16-apr-15 when medisense precision pcx glucose test strips are being run on the i-stat 1 analyzer, the glucose test strip data can be made available to qc manager data management program. Click the upload pcx strip data to qc manager 2.2 box to enable this function. ...
Page 333
Rev. Date: 16-apr-15 art: 714384-01f 22-13 security the security features allow for the creation of security profiles providing different levels of access to various areas and functions of the cds application. Individual users can then be assigned to a security profile, and then choose their own ind...
Page 334
22-14 art: 714384-01f rev. Date: 16-apr-15.
Page 335
Rev. Date: 16-apr-15 art: 714384-01f 22-15 interface program customization overview the central data station can output results to an external computer system such as an lis or his. The central data station also provides a function that, when enabled, will also transmit all of the precision pcx resu...
Page 336
22-16 art: 714384-01f rev. Date: 16-apr-15 procedure 1. The cds program must be running and the external interface must be enabled. 2. Double click the i-stat interface icon in the system tray (next to the clockinthelowerrighthandcornerofthescreen)toopentheinterface program’s main screen. 3. Click f...
Page 337
Rev. Date: 16-apr-15 art: 714384-01f 22-17 overview of the central data station program the central data station (cds) software includes the following point-of-care testing process management functions: • managinginstruments • managinganalyzer customization profiles • managingoperators • maintaining...
Page 338
22-18 art: 714384-01f rev. Date: 16-apr-15.
Page 339
Rev. Date: 16-apr-15 art: 714384-01f 22-19 administration tools overview thisworkspaceisusedto: • assign names to download locations, • assign instruments to locations, • configure the reporting and monitoring options for each instrument, and • set required download intervals for each location. The ...
Page 340
22-20 art: 714384-01f rev. Date: 16-apr-15 delete download location assignment click location delete download location assignment... From the menu or click delete assig. In the toolbar. Edit download location assignment click location edit download location assignment... From the menu or click e...
Page 341
Rev. Date: 16-apr-15 art: 714384-01f 22-21 select a location from the dropdown list. If the location is not listed, add the location using the instructions under add new download location assigment. If the location does not have a download device associated with it, such as an instrument used for tr...
Page 342
22-22 art: 714384-01f rev. Date: 16-apr-15 delete instrument clickthelocation/methodfortheinstrumenttobedeletedandthentheserial numberoftheinstrumenttobedeleted.Click instrument delete... From the menuorclick delete inst. In the toolbar. Move instrument clickthelocation/methodfortheinstrumenttobem...
Page 343
Rev. Date: 16-apr-15 art: 714384-01f 22-23 note: the i-stat 1 analyzer can also be customized either to warn the end usersthatadownloadisrequiredortolockoutendusersifthetimefor a download has been reached or exceeded. The download criteria for analyzersandforthecdsmonitorshouldbeselectedtomakesense....
Page 344
22-24 art: 714384-01f rev. Date: 16-apr-15 instrument log the instrument log tracks all changes made in the instruments tab page. Additional comments can be added to the log by clicking add note in the toolbar. Date range... Data can be viewed within a user defined default range or by a manually ent...
Page 345
Rev. Date: 16-apr-15 art: 714384-01f 22-25 operator workspace overview thisworkspaceisusedto: • recordoperatornamesandidentificationnumbers • recordcertificationdatesandcertificationexpirationdates • assignoperatorstodepartments • addcomments when the i-stat 1 analyzer is customized to use the opera...
Page 346
22-26 art: 714384-01f rev. Date: 16-apr-15 add operator this function is used to add new operators to the list of operators. Alternatively, operator lists can be imported (see operator list import at the end of this section).Click operator add... From menu or click on add in the toolbar. Enter the...
Page 347
Rev. Date: 16-apr-15 art: 714384-01f 22-27 update certifications select the operator or operators. Click operator update certification... From the menu or click update cert. In the toolbar and complete the update certification form. Add certification the add certification button allows operators w...
Page 348
22-28 art: 714384-01f rev. Date: 16-apr-15 edit department name clickthedepartmentnametoedit.Click operator edit department name fromthemenuorclick dept. Name in the toolbar. The unassigned designation cannot be changed. Operator and certification reports clickonthedownarrownextto reportintheopera...
Page 349
Rev. Date: 16-apr-15 art: 714384-01f 22-29 operator log the operator log tracks changes made and “add note” entries made in the operator tab page. The date range… button can be used to specify a time period to be viewed and the delete… button to delete entries. To print the log press the f2keyorsele...
Page 350
22-30 art: 714384-01f rev. Date: 16-apr-15.
Page 351
Rev. Date: 16-apr-15 art: 714384-01f 22-31 operator list import import list instructions 1. Under fields in text file:, use the mouse to drag and drop the field names so they match the order in which the fields appear in the text file containing the list to be imported. If a field does not appear in...
Page 352
22-32 art: 714384-01f rev. Date: 16-apr-15 7. If all operators are to be assigned to the same department, such as nursing or perfusion, click on assume a single department for all operators and enter or select the department from the drop down list. If this option is selected, the text file does not...
Page 353
Rev. Date: 16-apr-15 art: 714384-01f 22-33 database maintenance overview thisworkspaceallowsthedatabasetobebackedup,deletedandrestored.A "statistics" tab page also allows users to view a summary page of result types contained in the database. Archive test results backup test results: this function a...
Page 354
22-34 art: 714384-01f rev. Date: 16-apr-15 • location • comment • interfacecomment • serialnumber • department 7. Select a directory. 8. Selectamethodormethodstobackuporclickselect all methods. 9. Click the button marked backup or backup and delete and follow the prompts. Note:whenresultsarebeingdel...
Page 355
Rev. Date: 16-apr-15 art: 714384-01f 22-35 restore test results: this function allows test results that have been deleted fromthecdsbutbackedupelsewheretoberestoredtothedatabase. 1. Click on main open administration functions database maintenance. 2. Insertdiskorcdwherefilesarelocated. 3. Clickt...
Page 356
22-36 art: 714384-01f rev. Date: 16-apr-15 selecting an individual result type and then clicking on details allows you to viewasimilarstatisticalbreakdownforthatparticularresulttype: 1. The total number of that particular result type in the database, 2. The date and time of the oldest result of that...
Page 357
Rev. Date: 16-apr-15 art: 714384-01f 22-37 items inventory workspace the items tab is used to define the inventory items for the i-stat system and other point-of-care tests. To select an item available from i-stat and its distributors, highlight the item in the choose items from the listontherightsi...
Page 358
22-38 art: 714384-01f rev. Date: 16-apr-15 stock thestocktabincludesbothinventoryandestimatedinventorystatistics. Inventory: the number of given items as counted and entered by the user. The inventory is automatically updated when new orders are received under the orders tab. Estimated inventory: th...
Page 359
Rev. Date: 16-apr-15 art: 714384-01f 22-39 the estimated inventory for i-stat cartridges and medisense pcx glucose test strips will automatically begin updating with the next analyzer transmission. Clickthe refresh buttontoupdatetheworkspacefortransmitteddata.Boththe inventory and the estimated inve...
Page 360
22-40 art: 714384-01f rev. Date: 16-apr-15 clickthe add button to add another item or the delete button to delete an item. Clickthe receive all button to automatically enter items and quantities, as they were ordered. Use the delete button on the tool bar to delete an order. Use the find lot and fin...
Page 361
Rev. Date: 16-apr-15 art: 714384-01f 22-41 distribution use the add button in the tool bar to record the distribution of consumables. The item drop down menu includes all consumables entered in the items tab. The location drop down menu includes all locations entered in the instrument and locationwo...
Page 362
22-42 art: 714384-01f rev. Date: 16-apr-15 inventory log theinventorylogdocumentseachactiontakenintheitems,stock,distribution andorderstabs.Clickthe date range button in the toolbar to select the a defaultdaterangeorastartandenddateforthisreport.Clickthedeletebutton to delete entries in the log..
Page 363
Rev. Date: 16-apr-15 art: 714384-01f 22-43 customization workspace overview this workspace is used to create profiles with site specific test characteristics for the analyzers. See the customization section of this manual for details of items that can be customized and their default settings. Enabli...
Page 364
22-44 art: 714384-01f rev. Date: 16-apr-15 default customization profile the first step in customization is to create a default customization profile. This is the profile initially assigned to every new location. To change the default profile, use the directions under making selections or click the ...
Page 365
Rev. Date: 16-apr-15 art: 714384-01f 22-45 preferences for locations can also be changed by selecting an existing preference from the apply preferences submenu. Select the location or locations to be changed. Click profile apply preferences. Select the desiredpreferencesandclick apply.Clickview pr...
Page 366
22-46 art: 714384-01f rev. Date: 16-apr-15 unit set window details of each unit set are displayed under the analytes column. Details are also listed in the customization section in this manual. Tocreateauniqueunitset,click unitset99 and then the user settings tab. Then select the name and units for ...
Page 367
Rev. Date: 16-apr-15 art: 714384-01f 22-47 i-stat analyzer and philips bam clew windows a new clew is added to the window via the software update process twice a year. If the update clews feature is active, the default customization profile mustbeupdatedaftereachnewclewisadded.Clickthenewclewandclic...
Page 368
22-48 art: 714384-01f rev. Date: 16-apr-15 preferences window fordetaileddescriptionsofthepreferences,seesection9,customization.The preferenceswindowhasseventabpages.Clickthetabtodisplaythedesired page. The following conventions are used in the preferences pages: • enable/disableanoptionbyclickingth...
Page 369
Rev. Date: 16-apr-15 art: 714384-01f 22-49 chart page selection box the chart page selection box is part of a feature allowing users to customize the chart page on their i-stat 1 analyzers in order to capture user-defined information such as ventillator settings. See the "i-stat 1 analyzer chart pag...
Page 370
22-50 art: 714384-01f rev. Date: 16-apr-15.
Page 371
Rev. Date: 16-apr-15 art: 714384-01f 22-51 user administration workspace overview the user administration workspace is designed as a tool for system administrators. It allows administrators to manage security profiles (a set of security settings determining the access to different cds screens and fu...
Page 372
22-52 art: 714384-01f rev. Date: 16-apr-15 type in the name of the new security profile, then check off the different workspacesandfunctionsusersassignedtothatsecuritylevelwillbeallowed to access, and then click ok. The newly created security profile will then be added to the available security prof...
Page 373
Rev. Date: 16-apr-15 art: 714384-01f 22-53 password management passwords once all the security profiles are created, and all cds users are assigned to the appropriate profiles, the administrator should provide the users with their assigned user names. Their initial password is istat. When a user log...
Page 374
22-54 art: 714384-01f rev. Date: 16-apr-15 changing a password if a user is logged on to the cds application, they can choose to change their password at any time by clicking on tools change password. A dialog boxwillappearaskingthemtoentertheiroldpassword,aswellastheirnew password (twice). After ...
Page 375
Rev. Date: 16-apr-15 art: 714384-01f 22-55 data viewers data from instruments downloaded to the cds are viewed in the data viewers. Data downloaded from the i-stat 1 analyzers can be viewed in separate data viewers for results, qc codes, simulator, unsent results, control results, calibration verifi...
Page 376
22-56 art: 714384-01f rev. Date: 16-apr-15 refreshing the data data is received continuously by the cds. Updating the viewers with the continuous incoming stream of data would make viewing the data difficult. Therefore, new data is not added to a viewer until the refresh button is pressed. The windo...
Page 377
Rev. Date: 16-apr-15 art: 714384-01f 22-57 the selection of a shorter date range enhances the system performance by limiting the amount of information needing to be presented. It is always possible to expand the range to view results from earlier and then reset to a more limited default period. The ...
Page 378
22-58 art: 714384-01f rev. Date: 16-apr-15 a two-sided sort dialog will then appear, listing columns available for sorting on the left, and sort columns on the right. Simply click the listing under columns available for sorting that you wish to sort your data by, and then drag that column title to t...
Page 379
Rev. Date: 16-apr-15 art: 714384-01f 22-59 printing selected records withadatavieweropen,highlighttherecordstobeprinted,click record print selected recordsorclicktheprint toolbar button. Send selected records withadatavieweropen,highlighttherecordstobesent,click record send selected recordsorcli...
Page 380
22-60 art: 714384-01f rev. Date: 16-apr-15 electronic simulator viewer all electronic simulator results, both external and internal, are listed in chronological order with the newest result at the top of the screen. To view all simulator results together for each analyzer, click the serial number co...
Page 381
Rev. Date: 16-apr-15 art: 714384-01f 22-61 sent column values sent column value definition can the record be resent? No this is the initial value when a data record is entered into the database. Yes pending this value means that the record is in the queue waiting to be processed by the interface. No...
Page 382
22-62 art: 714384-01f rev. Date: 16-apr-15 data export a data export option is available in the following areas of the central data station application: a. Data viewers b. Reports c. Trend report, and d. The extended simulator report screen toaccessthisoptionfromanyofthedataviewersorreports,clickon ...
Page 383
Rev. Date: 16-apr-15 art: 714384-01f 22-63 monitors download monitor thedownloadmonitorquicklyidentifiesthedownloadstatusofalllocationsand any locations that have instruments out of download compliance. The upper portion of the monitor shows the last time an analyzer from the listedlocationswasdownl...
Page 384
22-64 art: 714384-01f rev. Date: 16-apr-15 interface monitor the interface monitor accessed via the menu bar functions with an interface installed by abbott point of care inc. To access the the interface monitor for an interfaceinstalledbyathirdparty,clickonthe interface manager button in the tray a...
Page 385
Rev. Date: 16-apr-15 art: 714384-01f 22-65 reports overview reports for managing the point-of-care testing process are available from the cds program. Three reports can be generated: reagent management, method competency and method compliance. These show information summarized by operator, location,...
Page 386
22-66 art: 714384-01f rev. Date: 16-apr-15 method compliance this is a report of exceptions of policy and procedure for cartridge testing by department, location or operator. This information is available when there is an interface to an external computer. Select a date range for the report. Select ...
Page 387
Rev. Date: 16-apr-15 art: 714384-01f 22-67 method competence thisisareportofqualitycheckcodeoccurrenceforcartridgesbydepartment, operator, location or analyzer. Select a date range for the report. Select a report by department, location, operator, or analyzer and then select all locations or all dep...
Page 388
22-68 art: 714384-01f rev. Date: 16-apr-15 system customization: central data station settings configuration of the cds can be viewed. Customization: i-stat analyzer settings customization profiles of the i-stat analyzers can be viewed. Autosend when enabled, data will be transmitted automatically f...
Page 389
Rev. Date: 16-apr-15 art: 714384-01f 22-69 help technical support phone number for your customer support representative. Windows operating system and language support windows version/ service pack windows language supported cds language(s) nt 4.0, sp6a english english 2000 professional, sp4 english ...
Page 390
22-70 art: 714384-01f rev. Date: 16-apr-15.
Page 391: Cartridge and Test
Cartridge and test information art: 714258-01o rev. Date: 15-aug-16 i-stat ® sensors are available in a variety of panel configurations. Sensors are contained in cartridges with microfluidic components and, in some cartridges, calibration solution. I-stat cartridges are used with the i-stat 1 analyz...
Page 392
Art: 714258-01o rev. Date: 15-aug-16 2 * the ctni, ck-mb, ß-hcgand bnp cartridges can only be used with the i-stat 1 analyzer bearing the symbol. Analysis time: • act cartridge: to detection of end point - up to 1000 sec (16.7 min) • pt/inr cartridge: to detection of end point – up to 300 sec (5 min...
Page 393
3 rev. Date: 15-aug-16 art: 714258-01o cartridges collection options syringes evacuated tubes capillary tubes directly from skin puncture cartridges which measure total ß-hcg • with sodium or lithium anticoagulant (syringe must be filled to labeled capacity) • without anticoagulant if tested within ...
Page 394
Art: 714258-01o rev. Date: 15-aug-16 4 expected values measured: test units reportable range reference range (arterial) (venous) sodium/na mmol/l (meq/l) 100 – 180 138 – 146 138 – 146 potassium/k mmol/l (meq/l) 2.0 – 9.0 3.5 – 4.9 3.5 – 4.9 chloride/cl mmol/l (meq/l) 65 – 140 98 – 109 98 – 109 gluco...
Page 395
5 rev. Date: 15-aug-16 art: 714258-01o test units reportable range reference range (arterial) (venous) b-type natriuretic peptide / bnp pg/ml (ng/l) 15 – 5000 total beta-human chorionic gonadotropin /ß-hcg iu/l 5.0 – 2000.0 calculated: test units reportable range reference range (arterial) (venous) ...
Page 396
Cartridge configurations and sample volume ec 8 + (65µl) sodium (na) potassium (k) chloride (cl) ph pco2 urea nitrogen (bun)/urea glucose (glu) hematocrit (hct) tco2* hco3* be* anion gap* (angap) hemoglobin* (hb) 6 + (65µl) sodium (na) potassium (k) chloride (cl) urea nitrogen (bun)/urea glucose (gl...
Page 397
Rev. Date: 15-jul-16 art: 714173-01p sodium/na sodium is measured by ion-selective electrode potentiometry. In the calculation of results for sodium, concentration is related to potential through the nernst equation. The i-stat system uses direct (undiluted) electrochemical methods. Values obtained ...
Page 398
Na - 2 art: 714173-01p rev. Date: 15-jul-16 the i-stat reference range for whole blood listed above is similar to reference ranges derived from serum or plasma measurements with standard laboratory methods. The reference range programmed into the analyzer and shown above is intended to be used as a ...
Page 399
Rev. Date: 15-jul-16 art: 714173-01p na - 3 slope 1.00 0.98 0.95 int’t -0.11 3.57 5.26 sy.X 1.17 1.04 1.53 xmin 126 120 124 xmax 148 148 148 r 0.865 0.937 0.838 cartridge comparison the performance characteristics of the sensors are equivalent in all cartridge configurations. System difference analy...
Page 400
Na - 4 art: 714173-01p rev. Date: 15-jul-16 note: 1) bromide has been tested at two levels: the clsi recommended level and a therapeutic plasma concentration level of 2.5 mmol/l. The latter is the peak plasma concentration associated with halothane anesthesia, in which bromide is released. Apoc has ...
Page 401
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 15-jul-16 art: 714173-01p na - 5 references 1. N.W. Tietz, e.L. Pruden, o...
Page 403
Rev. Date: 15-jul-16 art: 714174-01o potassium/k potassium is measured by ion-selective electrode potentiometry. In the calculation of results for potassium, concentration is related to potential through the nernst equation. The i-stat ® system uses direct (undiluted) electrochemical methods. Values...
Page 404
K - 2 art: 714174-01o rev. Date: 15-jul-16 the i-stat reference range for whole blood listed above is similar to reference ranges derived from serum or plasma measurements with standard laboratory methods. The reference range programmed into the analyzer and shown above is intended to be used as a g...
Page 405
Rev. Date: 15-jul-16 art: 714174-01o k - 3 method comparison (mmol/l or meq/l) beckman nova synchron kodak stat cx ® 3 ektachem™ 700 profile ® 5 n 189 142 192 sxx 0.060 0.031 0.065 syy 0.055 0.059 0.055 slope 0.97 1.06 0.99 int't 0.02 -0.15 -0.01 sy.X 0.076 0.060 0.112 xmin 2.8 3.0 2.8 xmax 5.7 9.2 ...
Page 406
K - 4 art: 714174-01o rev. Date: 15-jul-16 anesthesia, in which bromide is released. Apoc has not identified a therapeutic condition that would lead to levels consistent with the clsi recommended level. Bromide at a concentration of 37.5 mmol/l increased i-stat potassium results and the rate of pota...
Page 407
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 15-jul-16 art: 714174-01o k - 5 references 1. N.W. Tietz, e.L. Pruden, o....
Page 409
Rev. Date: 15-jul-16 art: 714175-01o chloride/cl chloride is measured by ion-selective electrode potentiometry. In the calculation of results for chloride, concentration is related to potential through the nernst equation. The i-stat system uses direct (undiluted) electrochemical methods. Values obt...
Page 410
Cl - 2 art: 714175-01o rev. Date: 15-jul-16 the i-stat reference range for whole blood listed above is similar to reference ranges derived from serum or plasma measurements with standard laboratory methods. The reference range programmed into the analyzer and shown above is intended to be used as a ...
Page 411
Rev. Date: 15-jul-16 art: 714175-01o cl - 3 method comparison (mmol/l or meq/l) beckman synchron cx ® 3 kodak ektachem 700 nova stat profile ® 5 n 189 142 192 sxx 1.27 0.41 0.89 syy 0.88 0.90 0.88 slope 0.99 0.88 0.93 int’t -0.82 14.6 4.3 sy.X 1.65 1.84 2.33 xmin 93 63 96 xmax 114 128 117 r 0.817 0...
Page 412
Cl - 4 art: 714175-01o rev. Date: 15-jul-16 notes: 1) acetylcysteine has been tested at two levels: the clsi recommended level and a concentration of 0.30 mmol/l. The latter is 3 times the peak plasma therapeutic concentration associated with treatment to reverse acetaminophen poisoning. Apoc has no...
Page 413
Rev. Date: 15-jul-16 art: 714175-01o cl - 5 references 1. N.W. Tietz, e.L. Pruden, o. Siggaard-andersen, ”electrolytes ” in tietz textbook of clinical chemistry— second edition, c.A. Burtis and e.R. Ashwood, eds. (philadelphia: w.B. Saunders company, 1994). 2. D.S. Young, effects of drugs on clinica...
Page 414
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 15-jul-16 art: 714175-01o cl - 6.
Page 415
Rev. Date:15-jul-16 art: 714176-01q bun/urea urea is hydrolyzed to ammonium ions in a reaction catalyzed by the enzyme urease. Urease urea + h 2 o + 2h + 2nh + 4 + co 2 the ammonium ions are measured potentiometrically by an ion-selective electrode. In the calculation of results for urea, concentrat...
Page 416
Bun - 2 art: 714176-01q rev. Date: 15-jul-16 to convert a bun result in mg/dl to a urea result in mmol/l, multiply the bun result by 0.357. To convert a urea result in mmol/l to a urea result in mg/dl, multiply the mmol/l result by 6. To convert a urea result in mg/dl to a urea result in g/l, divide...
Page 417
Rev. Date: 15-jul-16 art: 714176-01q bun - 3 method comparison (mg/dl) beckman coulter lx20 ® dade dimension rxl-xpand ® beckman coulter cx9 ® n 39 32 26 sxx 0.36 0.48 0.39 syy 0.67 0.34 0.60 slope 1.03 1.05 1.00 int’t 1.39 -0.28 -0.38 sy.X 0.99 0.31 0.85 xmin 5 5 7 xmax 70 38 66 r 0.997 0.998 0.997...
Page 418
Bun - 4 art: 714176-01q rev. Date: 15-jul-16 thiocyanate 6.9 notes: 1) bromide has been tested at two levels: the clsi recommended level and a therapeutic plasma concentration of 2.5 mmol/l. The latter is the peak plasma concentration associated with halothane anesthesia, in which bromide is release...
Page 419
Rev. Date: 15-jul-16 art: 714176-01q bun - 5 references 1. D.S. Young, effects of drugs on clinical laboratory tests, 3rd ed. (washington, dc: american association of clinical chemistry, 1990). 2. B.E. Statland, clinical decision levels for lab tests (oradell, nj: medical economic books, 1987). 3. C...
Page 420
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Bun - 6 art: 714176-01q rev. Date: 15-jul-16.
Page 421
Rev. Date: 15-jul-16 art: 714177-01r glucose/glu glucose is measured amperometrically. Oxidation of glucose, catalyzed by the enzyme glucose oxidase, produces hydrogen peroxide (h 2 o 2 ). The liberated hydrogen peroxide is oxidized at the electrode to produce a current which is proportional to the ...
Page 422
Glu - 2 art: 714177-01r rev. Date: 15-jul-16 expected values reportable reference test/abbreviation units* range range 2 glucose/glu mg/dl 20 – 700 70 – 105 (fasting) mmol/l 1.1 – 38.9 3.9 – 5.8 g/l 0.20 – 7.00 0.70 – 1.05 * the i-stat system can be configured with the preferred units. To convert a ...
Page 423
Rev. Date: 15-jul-16 art: 714177-01r glu - 3 precision data (mg/dl) aqueous control mean sd %cv level 1 41.8 0.68 1.6 level 3 289 2.4 0.8 method comparison (mg/dl) beckman coulter lx20 bayer 860 dade dimension rxl-xpand n 35 40 32 sxx 2.21 4.71 0.98 syy 0.69 0.96 0.59 slope 1.03 0.99 1.01 int’t -3.3...
Page 424
Glu - 4 art: 714177-01r rev. Date: 15-jul-16 dopamine 0.006 formaldehyde 0.133 10 β–hydroxybutyrate 6.0 13 lactate 6.6 maltose 13.3 pyruvate 0.31 salicylate 4.34 thiocyanate (therapeutic) 0.5 15 uric acid 1.4 notes: 1) acetaminophen has been shown to interfere with glucose results in the i-stat 6+, ...
Page 425
Rev. Date: 15-jul-16 art: 714177-01r glu - 5 references 1. D.S. Young, effects of drugs on clinical laboratory tests, 3rd ed. (washington, dc: american asso- ciation of clinical chemistry, 1990). 2. P.C. Painter, j.Y. Cope, j.L. Smith, “reference ranges, table 41-20” in tietz textbook of clinical ch...
Page 426
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 15-jul-16 art: 714177-01r glu - 6.
Page 427
Rev. Date: 15-aug-16 art: 714178-01o hematocrit/hct and calculated hemoglobin/hb hematocrit is determined conductometrically. The measured conductivity, after correction for electrolyte concentration, is inversely related to the hematocrit. See below for information on factors affecting results. Cer...
Page 428
Hct - 2 art: 714178-01o rev. Date: 15-aug-16 to convert a result from %pcv to fraction packed cell volume, divide the %pcv result by 100. For the measurement of hematocrit, the i-stat system can be customized to agree with methods calibrated by the microhematocrit reference method using either k 3 e...
Page 429
Rev. Date: 15-aug-16 art: 714178-01o hct - 3 method comparison (%pcv) coulter ® s plus nova stat profile ® 5 abbott cell-dyn 4000 sysmex se9500 n 142 192 29 29 sxx 0.50 0.46 0.41 0.53 syy 1.09 1.31 0.77 0.76 slope 0.98 1.06 1.06 1.11 int’t 1.78 -3.98 -1.42 -4.19 sy.X 2.03 2.063 1.13 0.98 xmin 18 21 ...
Page 430
Hct - 4 art: 714178-01o rev. Date: 15-aug-16 sodium the sample electrolyte concentration is used to correct the measured conductivity prior to reporting hematocrit results. Factors that affect sodium will therefore also affect hematocrit. Bromide bromide (37.5 mmol/l) is known to result in an increa...
Page 431
Rev. Date: 15-aug-16 art: 714178-01o hct - 5 references 1. D.S. Young, effects of drugs on clinical laboratory tests, 3rd ed. (washington, dc: american association of clinical chemistry, 1990). 2. Clsi. Procedure for determining packed cell volume by the microhematocrit method; approved standard - t...
Page 432
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Hct - 6 art: 714178-01o rev. Date: 15-aug-16.
Page 433
Ionized calcium/ica rev. Date: 15-jul-16 art: 714179-01p ionized calcium is measured by ion-selective electrode potentiometry. In the calcu lation of results for ionized calcium concentration is related to potential through the nernst equation. Results are measured at 37°c. See below for information...
Page 434
Ica - 2 art: 714179-01p rev. Date: 15-jul-16 *the i-stat system can be configured with the preferred units. To convert a result from mmol/l to mg/dl, multiply the mmol/l value by 4. To convert mmol/l to meq/l multiply the mmol/l value by 2. The reference range programmed into the analyzer and shown ...
Page 435
Rev. Date: 15-jul-16 art: 714179-01p ica - 3 method comparison (mmol/l) radiometer ica1 nova stat profile n 47 57 sxx 0.009 0.017 syy 0.017 0.017 slope 0.925 0.960 int’t 0.113 0.062 sy.X 0.035 0.029 xmin 0.46 0.53 xmax 2.05 2.05 r 0.982 0.982 cartridge comparison the performance characteristics of t...
Page 436
Ica - 4 art: 714179-01p rev. Date: 15-jul-16 interference studies were based on clsi guideline ep7-a2. 6 test concentrations used were as per the clsi guideline unless otherwise indicated. When added to a plasma pool, the following substances (at the concentration indicated) were found to interfere ...
Page 437
Rev. Date: 15-jul-16 art: 714179-01p ica - 5 decrease ionized calcium results by approximately 0.03 mmol/l. 5) nithiodote (sodium thiosulfate) is indicated for the treatment of acute cyanide poisoning. The journal article titled “falsely increased chloride and missed anion gap elevation during treat...
Page 438
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Ica - 6 art: 714179-01p rev. Date: 15-jul-16 references 1. D.S. Young, effects of dr...
Page 439
Rev. Date: 01-jul-13 art: 714180-01l po 2 and calculated oxygen saturated/so 2 po 2 is measured amperometrically. The oxygen sensor is similar to a conventional clark electrode. Oxygen permeates through a gas permeable membrane from the blood sample into an internal electrolyte solution where it is ...
Page 440
Po2 - 2 art: 714180-01l rev. Date: 01-jul-13 clinical significance po 2 (partial pressure of oxygen) is a measurement of the tension or pressure of oxygen dissolved in blood. Some causes for decreased values of po 2 include decreased pulmonary ventilation (e.G., airway obstruction or trauma to the b...
Page 441
Rev. Date: 01-jul-13 art: 714180-01l po2 - 3 method comparison data were collected using clsi guideline ep9-a. 4 arterial blood samples were collected from hospital patients in 3 cc blood gas syringes and were analyzed in duplicate on the i-stat system and the comparative method within 5 minutes of ...
Page 442
Abbott point of care inc. Abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc.. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 714180-01l po2 - 4 references 1. D.S. Young,...
Page 443
Rev. Date: 15-jul-16 art: 714181-01n ph ph is measured by direct potentiometry. In the calculation of results for ph, concen tra tion is related to potential through the nernst equation. Results are reported at 37°c. See below for information on factors affecting results. Certain substances, such as...
Page 444
Ph - 2 art: 714181-01n rev. Date: 15-jul-16 the reference range programmed into the analyzer and shown above is intended to be used as a guide for the interpretation of results. Since reference ranges may vary with demographic factors such as age, gender and heritage, it is recommended that referenc...
Page 445
Rev. Date: 15-jul-16 art: 714181-01n ph - 3 precision data aqueous control mean sd %cv level 1 7.165 0.005 0.08 level 3 7.656 0.003 0.04 method comparison radiometer nova radiometer il bge ica 1 stat profile 5 abl500 n 62 47 57 45 sxx 0.005 0.011 0.006 0.004 syy 0.009 0.008 0.008 0.008 slope 0.974 1...
Page 446
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 15-jul-16 art: 714181-01n ph - 4 references 1. D.S. Young, effects of dru...
Page 447
Rev. Date: 01-jul-13 art: 714182-01r pco 2 and calculated values for hco 3 , base excess and anion gap pco 2 is measured by direct potentiometry. In the calculation of results for pco 2 , concen tration is related to potential through the nernst equation. Results are reported at 37°c. Calculated val...
Page 448
Pco2 - 2 art: 714182-01r rev. Date: 01-jul-13 expected values reportable reference test/abbreviation units* range range (arterial) (venous) partial pressure carbon dioxide/ pco 2 mmhg 5 – 130 35 – 45 3 41 – 51 kpa 0.67 – 17.33 4.67 – 6.00 5.47 – 6.80 bicarbonate/hco 3 mmol/l 1.0 – 85.0 22 – 26** 23 ...
Page 449
Rev. Date: 01-jul-13 art: 714182-01r pco2 - 3 temperature “correction” algorithm pco 2 is a temperature-dependent quantity and is measured at 37°c. The pco 2 reading at a body temperature other than 37°c can be ‘corrected’ by entering the patient’s temperature on the chart page of the analyzer. See ...
Page 450
Pco2 - 4 art: 714182-01r rev. Date: 01-jul-13 method comparison (mmhg) pco 2 il bge pco 2 radiometer abl500 n 62 29 sxx 0.69 0.74 syy 1.24 0.53 slope 1.003 1.016 int’t -0.8 1.1 sy.X 1.65 0.32 xmin 30.4 28 xmax 99.0 91 r 0.989 0.999 factors affecting results* exposing the sample to air allows co 2 to...
Page 451
Rev. Date: 01-jul-13 art: 714182-01r pco2 - 5 references 1. Clsi. Blood gas and ph analysis and related measurements; approved guideline. Clsi document c46-a [isbn 1-56238-444-9]. Clsi, 940 west valley road, suite 1400, wayne, pennsylvania 19087-1898, usa 2001. 2. D.S. Young, effects of drugs on cli...
Page 452
Abbott point of care inc. Abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 714182-01r pco2 - 6.
Page 453
Total carbon dioxide/ (tco 2 ) rev. Date: 01-jul-13 art: 716661-01g intended use the test for tco 2 , as part of the i-stat system, is intended for use in the in vitro quantification of total carbon dioxide in arterial, venous, or capillary whole blood. Carbon dioxide is used in the diagnosis, monit...
Page 454
Tco2 - 2 art: 716661-01g rev. Date: 01-jul-13 the reference ranges programmed into the analyzer and shown above are intended to be used as guides for the interpretation of results. Since reference ranges may vary with demographic factors such as age, gender, and heritage, it is recommended that refe...
Page 455
Rev. Date: 01-jul-13 art: 716661-01g tco2 - 3 precision data (mmol/l) aqueous control mean sd %cv level 1 17.4 0.62 3.6 level 3 34.6 0.62 1.8 method comparison (mmol/l) tco 2 (calculated) il bge tco 2 (calculated) beckman coulter cx ® 3 tco 2 (measured) beckman coulter lx ® 20 n 62 51 35 sxx 0.40 0....
Page 456
Abbott point of care inc. Abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 716661-01g tco2 - 4 references 1. Ifcc refere...
Page 457
Rev. Date: 15-jul-16 art: 714183-01w creatinine/crea creatinine is measured amperometrically. Creatinine is hydrolyzed to creatine in a reaction catalyzed by the enzyme creatinine amidohydrolase. Creatine is then hydrolyzed to sarcosine in a reaction catalyzed by the enzyme creatine amidinohydrolase...
Page 458
Crea - 2 art: 714183-01w rev. Date: 15-jul-16 plasma fraction of arterial, venous, or capillary whole blood (dimension µmol l -1 ) for in vitro diagnostic use. Creatinine values assigned to i-stat’s controls and calibration verification materials are traceable to the u.S. National institute of stand...
Page 459
Rev. Date: 15-jul-16 art: 714183-01w crea - 3 *the usual warning relating to the use of regression analysis is summarized here as a reminder. For any analyte, “if the data are collected over a narrow range, the estimate of the regression parameters is relatively imprecise and may be biased. Therefor...
Page 460
Crea - 4 art: 714183-01w rev. Date: 15-jul-16 the following substances are known not to significantly interfere with the i‑stat creatinine assay at the stated test concentrations: substance test concentration (mmol/l) acetaldehyde 0.045 9 acetaminophen (therapeutic) 0.132 9 acetylcysteine (therapeut...
Page 461
Rev. Date: 15-jul-16 art: 714183-01w crea - 5 5) bromide has been tested at two levels: the clsi recommended level and a therapeutic plasma concentration level of 2.5 mmol/l. The latter is the peak plasma concentration associated with halothane anesthesia, in which bromide is released. Apoc has not ...
Page 462
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Crea - 6 art: 714183-01w rev. Date: 15-jul-16 references 1. D. S. Young. Effects of ...
Page 463
Rev. Date: 15-jul-16 art: 714184-01o lactate/lac lactate is measured amperometrically. The enzyme lactate oxidase, immobilized in the lactate biosensor, selectively converts lactate to pyruvate and hydrogen peroxide (h 2 o 2 ). The liberated hydrogen peroxide is oxidized at a platinum electrode to p...
Page 464
Lac - 2 art: 714184-01o rev. Date: 15-jul-16 fluka, >99 % purity). I-stat system controls and calibration verification materials are validated for use only with the i-stat system and assigned values may not be commutable with other methods. Further information regarding metrological traceability is ...
Page 465
Rev. Date: 15-jul-16 art: 714184-01o lac - 3 precision data aqueous control n mean sd %cv (mmol/l) level 1 120 6.35 0.08 1.21 level 3 120 0.81 0.03 3.27 method comparison radiometer abl 725 hitachi 917 (mmol/l) (whole blood vs. (i-stat whole blood vs. Whole blood) hitachi plasma) n 47 47 sxx 0.123 0...
Page 466
Lac - 4 art: 714184-01o rev. Date: 15-jul-16 notes: 1) hydroxyurea is a dna synthesis inhibitor used in the treatment of various forms of cancer, sickle cell anemia, and hiv infection. This drug is used to treat malignancies including melanoma, metastatic ovar- ian cancer, and chronic myelogenous le...
Page 467
Rev. Date: 15-jul-16 art: 714184-01o lac - 5 references 1. D.S. Young, effects of drugs on clinical laboratory tests, 3rd ed. (washington, dc: american association of clinical chemistry, 1990). 2. D.B. Sacks, carbohydrates, in tietz textbook of clinical chemistry, second edition, ed. C.A. Burtis and...
Page 468
Abbott point of care inc. 100 and 200 abbott park road abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh the hague the netherlands ©2016 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 15-jul-16 art: 714184-01o lac - 6 20. Charles r.A, bee y.M, eng p.H.K., go...
Page 469
Celite activated clotting time/ ( celite act) rev. Date: 01-jul-13 art: 714185-01p the i-stat ® celite ® activated clotting time test, celite act, is a measure of the time required for complete activation of the coagulation cascade. 1 in traditional act tests, coagulation is initiated by mixing a wh...
Page 470
Celite act - 2 art: 714185-01p rev. Date: 01-jul-13 contents each i-stat celite act cartridge provides a sample collection chamber, sensors to detect the coagulation endpoint, and dry reagents necessary to initiate and allow coagulation. Stabilizers and reagents are coated on a section of the sensor...
Page 471
Rev. Date: 01-jul-13 art: 714185-01p celite act - 3 method comparison data were collected using a modification of the clsi guideline ep9-a. 2 venous or arterial blood samples were collected in plastic syringes and analyzed in duplicate on the i-stat system and in duplicate using the comparative meth...
Page 472
Celite act - 4 art: 714185-01p rev. Date: 01-jul-13 factors affecting results* *it is possible that other interfering substances may be encountered. These results are representative and your results may differ somewhat due to test-to-test variation. The degree of interference at concentrations other...
Page 473
Rev. Date: 01-jul-13 art: 714185-01p celite act - 5 the graphs below indicate the response of the same five donors with respect to the act result on the medtronic hr-act and the hemochron celite ftca 510..
Page 474
Celite act - 6 art: 714185-01p rev. Date: 01-jul-13 performance of the i-stat celite act at lower levels of heparin is shown below with two “low range” act methods included for comparison: medtronic lr-act, s test limitations the i-stat celite act test is to be used with fresh venous or arterial who...
Page 475
Rev. Date: 01-jul-13 art: 714185-01p celite act - 7 the analyzer should remain on a level surface with the display facing up during testing. If the analyzer is not level, the act result may be affected by more than 10 %. A level surface includes running the handheld in the downloader/recharger. Hemo...
Page 476
Abbott point of care inc. Abbottpark,il60064•usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 714185-01p celite act - 8 references 1. Hattersly,...
Page 477
Kaolin activated clotting time/ ( kaolin act) rev. Date: 01-jul-13 art: 715878-01m the i-stat ® kaolin activated clotting time test, kaolin act, is a measure of the time required for complete activation of the coagulation cascade. 1 in traditional act tests, coagulation is initiated by mixing a whol...
Page 478
Kaolin act - 2 art: 715878-01m rev. Date: 01-jul-13 contents each i-stat kaolin act cartridge provides a sample collection chamber, sensors to detect the coagulation endpoint, and dry reagents necessary to initiate and allow coagulation. Stabilizers and reagents are coated on a section of the sensor...
Page 479
Rev. Date: 01-jul-13 art: 715878-01m kaolin act - 3 method comparison data were collected using a modification of the clsi guideline ep9-a. 2 venous or arterial blood samples were collected in plastic syringes and analyzed in duplicate on the i-stat system and in duplicate using the comparative meth...
Page 480
Kaolin act - 4 art: 715878-01m rev. Date: 01-jul-13.
Page 481
Rev. Date: 01-jul-13 art: 715878-01m kaolin act - 5 the following two graphs indicate the response of the same three donors with respect to the act result on the medtronic hr-act and the hemochron kaolin ftk-act..
Page 482
Kaolin act - 6 art: 715878-01m rev. Date: 01-jul-13 test limitations the i-stat kaolin act test is to be used with fresh venous or arterial whole blood samples. The presence of exogenously added heparin, citrate, oxalate, or edta will interfere with test results. Poor technique in sample collection ...
Page 483
Rev. Date: 01-jul-13 art: 715878-01m kaolin act - 7 specimen collection and preparation the i-stat kaolin act test can be performed using venous or arterial samples. Venipunctures and arterial punctures • collectiontechniqueresultingingoodbloodflowmustbeused. • thesamplefortestingshouldbedrawnintoa ...
Page 484
Abbott point of care inc. Abbottpark,il60064•usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 715878-01m kaolin act - 8.
Page 485
Prothrombin time/ (pt/inr) rev. Date: 01-jul-13 art: 715236-01m the i-stat ® pt/inr test is a whole blood determination of the prothrombin time used for monitoring oral anticoagulant (coumadin or warfarin) therapy. The test determines the time required for complete activation of the extrinsic pathwa...
Page 486
Pt/inr - 2 art: 715236-01m rev. Date: 01-jul-13 intended use the i-stat pt, a prothrombin time test, is useful for monitoring patients receiving oral anticoagulation therapy such as coumadin or warfarin. Contents each i-stat pt/inr cartridge provides a sample collection chamber, sensors to detect th...
Page 487
Rev. Date: 01-jul-13 art: 715236-01m pt/inr - 3 the below imprecision data for lyophilized plasma control material were collected during studies at an abbott point of care facility and during clinical trials. Sd and %cv are typical of current performance. Current value assignment sheets should be re...
Page 488
Pt/inr - 4 art: 715236-01m rev. Date: 01-jul-13 i-s tat pt (inr) siemens system ® ca 1500 pt (inr) i-s tat pt (inr) sta compact ® pt (inr) siemens sysmex ® ca 1500 pt (inr).
Page 489
Rev. Date: 01-jul-13 art: 715236-01m pt/inr - 5 data is presented below from one clinical site comparing data from capillary samples to data from venous samples analyzed on the i-stat system. Statistic capillary vs. Venous n 39 mean (inr) 2.4 range (inr) 1.3 – 5.4 sx (inr) 0.960 slope 1.049 intercep...
Page 490
Pt/inr - 6 art: 715236-01m rev. Date: 01-jul-13 factors affecting results • the presence of exogenously added heparin, citrate, oxalate, or edta from blood collection devices will interfere with test results. • poor technique in sample collection may compromise the results. (see specimen collection ...
Page 491: Ec8+
Rev. Date: 01-jul-13 art: 715236-01m pt/inr - 7 specimen collection and preparation caution: the i-stat pt/inr cartridge is designed to accept a sample between 20 and 45 microliters. A single drop of blood from either a finger puncture or as formed at the tip of a syringe will typically be within th...
Page 492
Abbott point of care inc. Abbottpark,il60064•usa emergo europe molenstraat 15 2513 bh the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 715236-01m pt/inr - 8 references 1. Kirkwood tbl. C...
Page 493
Cardiac troponin i/ (ctni) rev. Date: 01-jul-13 art: 715595-01r intended use the i-stat ® cardiac troponin i (ctni) test is an in vitro diagnostic test for the quantitative measurement of cardiac troponin i (ctni) in whole blood or plasma. Measurements of cardiac troponin i are used in the diagnosis...
Page 494
Ctni - 2 art: 715595-01r rev. Date: 01-jul-13 metrological traceability the i-stat system test for cardiac troponin-i (ctni) measures cardiac troponin-i amount-of-substance concentration in plasma or the plasma fraction of whole blood (dimension ng ml -1 ) for in vitro diagnostic use. Cardiac tropon...
Page 495
Rev. Date: 01-jul-13 art: 715595-01r ctni - 3 exam) and ecg should be used in conjunction with troponin in the diagnostic evaluation of suspected myocardial infarction. 3 a serial sampling protocol is recommended to facilitate the identification of temporal changes in troponin levels characteristic ...
Page 496
Ctni - 4 art: 715595-01r rev. Date: 01-jul-13 method comparison (ng/ml) dade behring stratus ® cs n 189 sxx 0.28 syy 0.31 slope 0.883 int’t 0.029 sy.X 1.40 xmin 0.00 xmax 46.27 r 0.975 analytical and functional sensitivities the analytical sensitivity of the ctni method is 0.02 ng/ml, which is the l...
Page 497
Rev. Date: 01-jul-13 art: 715595-01r ctni - 5 analytical specificity the ctni method is specific for cardiac troponin i. The following muscle proteins were tested and found to have an insignificant effect on the measured ctni. Crossreactant concentration percent crossreactivity troponin c (cardiac) ...
Page 498
Ctni - 6 art: 715595-01r rev. Date: 01-jul-13 samples from patients who have been exposed to animals or who have received therapeutic or diagnostic procedures employing immunoglobulins or reagents derived from immunoglobulins may contain antibodies, e.G., hama or other heterophile antibodies, which ...
Page 499
Rev. Date: 01-jul-13 art: 715595-01r ctni - 7 interference testing the following substances were found to have no significant effect (less than 10%) on the ctni method, when added to a plasma pool containing approximately 2 ng/ml of cardiac troponin i, at the concentrations indicated: compound test ...
Page 500
Ctni - 8 art: 715595-01r rev. Date: 01-jul-13 references 1. Braunwald, e, et al. Acc/aha 2002 guideline update for the management of patients with unstable angina and non-st-segment elevation myocardial infarction: a report of the american college of cardiology/american heart association task force ...
Page 501
Rev. Date: 01-jul-13 art: 715595-01r ctni - 9 1898 usa, 2007. 19. Bjerner et al. Immunometric assay interference: incidence and prevention. Clin. Chem. 2002; 48:613. 20. Kricka, interferences in immunoassays – still a threat. Clin. Chem. 2000; 46:1037. 21. Schroff et al. Human anti-murine immunoglob...
Page 502
Abbott point of care inc. Abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 715595-01r ctni - 10
Page 503
Creatine kinase mb/ (ck-mb) rev. Date: 01-jul-13 art: 716675-01l intended use the i-stat ® ck-mb test is an in vitro diagnostic test for the quantitative measurement of creatine kinase mb mass in whole blood or plasma samples. Ck-mb measurements can be used as an aid in the diagnosis and treatment o...
Page 504
Ck-mb - 2 art: 716675-01l rev. Date: 01-jul-13 metrological traceability the i-stat system test for creatine kinase-mb (ck-mb) measures creatine kinase-mb amount-of- substance concentration mass in plasma or the plasma fraction of venous whole blood (dimension ng/ml) for in vitro diagnostic use. Cre...
Page 505
Rev. Date: 01-jul-13 art: 716675-01l ck-mb - 3 the european society of cardiology / american college of cardiology consensus document notes that in the clinical setting of a reinfarction, ck-mb may be more useful for monitoring for mi than cardiac troponin i (ctni) or cardiac troponin t (ctnt) becau...
Page 506
Ck-mb - 4 art: 716675-01l rev. Date: 01-jul-13 method comparison (ng/ml) abbott axsym n 263 sxx 1.84 syy 2.66 slope 1.01 int’t -0.19 sy.X 3.98 xmin 0.04 xmax 224 r 0.994 analytical sensitivities the sensitivity of the ck-mb method is 0.6 ng/ml, which is the lowest ck-mb level that can be distinguish...
Page 507
Rev. Date: 01-jul-13 art: 716675-01l ck-mb - 5 whole blood sample concentration diluted concentration % recovery (ng/ml) (ng/ml) a 73.24 40.73 108.7% b 8.90 6.07 101.5% c 47.74 26.91 109.3% plasma sample concentration diluted concentration % recovery (ng/ml) (ng/ml) a 73.24 — — b 8.90 — — c 47.74 — ...
Page 508
Ck-mb - 6 art: 716675-01l rev. Date: 01-jul-13 interference testing when added to a plasma pool containing approximately 20 ng/ml of creatine kinase mb isoenzyme, the following substances were found to have no significant effect (less than 10%) on the ck-mb method at the concentrations indicated com...
Page 509
Rev. Date: 01-jul-13 art: 716675-01l ck-mb - 7 references 1. Braun wald, e, et al. Acc/aha 2002 guideline update for the management of patients with unstable angina and non-st-segment elevation myocardial infarction: a report of the american college of cardiology/ american heart association task for...
Page 510
Abbott point of care inc. Abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 716675-01l ck-mb - 8.
Page 511
B-type natriuretic peptide/ (bnp) rev. Date: 01-jul-13 art: 716969-01h intended use the i-stat ® bnp test is an in vitro diagnostic test for the quantitative measurement of b-type natriuretic peptide (bnp) in whole blood or plasma samples using edta as the anticoagulant. Bnp measurements can be used...
Page 512
Bnp - 2 art: 716969-01h rev. Date: 01-jul-13 metrological traceability the i-stat system test for b-type natriuretic peptide (bnp) measures bnp amount-of-substance concentration in plasma or the plasma fraction of edta anticoagulated whole blood (units of measure: pg/ml or ng/l) for in vitro diagnos...
Page 513
Rev. Date: 01-jul-13 art: 716969-01h bnp - 3 included the use of natriuretic peptide (e.G., bnp) testing along with electrocardiography and chest x-rays in their guidelines for the diagnosis or rule out of hf. 14 the breathing not properly study, a 1586 patient multinational prospective study, valid...
Page 514
Bnp - 4 art: 716969-01h rev. Date: 01-jul-13 expected values non-heart failure population plasma samples from 890 individuals (465 females, 425 males) who had not been diagnosed with heart failure were tested with the axsym ® bnp assay. This population included non-hospitalized patients with renal d...
Page 515
Rev. Date: 01-jul-13 art: 716969-01h bnp - 5 non-heart failure population - females (age group) all years 45-54 years 55-64 years 65-74 years 75+ years sample size (n=) 465 98 75 77 133 82 median (pg/ml) 26 23 23 37 23 25 mean (pg/ml) 46 34 34 51 46 69 sd (pg/ml) 70 37 36 48 63 126 95th percentile 1...
Page 516
Bnp - 6 art: 716969-01h rev. Date: 01-jul-13 heart failure population - males nyha functional class all i ii iii iv sample size (n=) 462 94 215 121 32 median (pg/ml) 268 122 258 293 1645 mean (pg/ml) 524 314 409 597 1646 sd (pg/ml) 719 390 539 821 1032 5th percentile 12 9 14 22 265 95th percentile 1...
Page 517
Rev. Date: 01-jul-13 art: 716969-01h bnp - 7 a box and whiskers plot of the clinical study population, broken down by nyha classification, is presented in the following graph. The dashed line represents 100 pg/ml, the suggested decision threshold for the axsym bnp assay. In support of previous liter...
Page 518
Bnp - 8 art: 716969-01h rev. Date: 01-jul-13 the i-stat bnp calibrators are traceable to an internal reference standard that has been prepared gravimetrically with synthetic bnp. The internal reference standard underwent a one-time value assignment to align with the architect bnp assay with a decisi...
Page 519
Rev. Date: 01-jul-13 art: 716969-01h bnp - 9 was measured in 10 i-stat bnp cartridges from a single cartridge lot; three lots of cartridges were employed. The mean within-sample bnp concentration ranged from 84 – 3925 pg/ml and the within- sample imprecision (%cv) ranged from 3.4 to 9.4%; the averag...
Page 520
Bnp - 10 art: 716969-01h rev. Date: 01-jul-13 equivalence of whole blood and plasma (x-axis) plasma([bnp] plasma([bnp] n 49 36 mean (pg/ml) 776 146 sxx (pg/ml) 122.0 18.5 syy (pg/ml) 98.1 16.5 slope 0.946 1.01 intercept 50.2 -0.2 sy.X 107.3 28.3 xmin 0 0 xmax 4173 922 correlation,r 0.997 0.996 analy...
Page 521
Rev. Date: 01-jul-13 art: 716969-01h bnp - 11 plasma blood sample concentration pg/ml) diluted concentration (pg/ml) % recovery a 590 — — b 2764 — — c 5123 — — a+b — 1570 94% b+c — 3992 101% a+c — 2734 96% a plasma sample was spiked with bnp to a value of approximately 5000 pg/ml and the concentrati...
Page 522
Bnp - 12 art: 716969-01h rev. Date: 01-jul-13 algorithms designed to detect their effects, the possibility of interference causing erroneous results should be evaluated carefully in cases where there are inconsistencies in the clinical information. Partially clotted samples can result in elevated bn...
Page 523
Rev. Date: 01-jul-13 art: 716969-01h bnp - 13 interference testing the following substances were found to have no significant effect (less than 10%) on the bnp method, when added to a plasma pool containing approximately 1000 pg/ml of b-type natriuretic peptide at the concentrations indicated: inter...
Page 524
Bnp - 14 art: 716969-01h rev. Date: 01-jul-13 references 1. Maisel a, mehra mr. Understanding b-type natriuretic peptide and its role in diagnosing and monitoring congestive heart failure, clin cornerstone 2005, 7 suppl 1: s7-17. 2. Senni m, tribouilloy cm, rodeheffer rj, et al. Congestive heart fai...
Page 525
Rev. Date: 01-jul-13 art: 716969-01h bnp - 15 17. Rodeheffer rj. Measuring plasma b-type natriuretic peptide in heart failure: good to go in 2004? J am coll cardiol. 2004 aug 18; 44(4): 740-9. 18. Doust ja, petrzak e, dobson a, glasziou p. How well does b-type natriuretic peptide predict death and c...
Page 526
Abbott point of care inc. Abbott park, il 60064 • usa emergo europe molenstraat 15 2513 bh, the hague the netherlands tel: (31)70 345 8570 fax: (31)70 346 7299 ©2013 abbott point of care inc. All rights reserved. Printed in usa. Rev. Date: 01-jul-13 art: 716969-01h bnp - 16 36. Clinical and laborato...
Page 527: Total Beta-Human
Total beta-human chorionic gonadotropin (β-hcg) art:730474-01d rev.Date: 24-jan-17 arial regular,helveticaneue 55roman,helveticaneue 56italic,helveticaneue 7 6 bolditalic,helveticaneue 95black helveticaneue 55roman,helveticaneue 56italic,helveticaneue 7 5 b o l d , h e l v e t i c a neue76bolditalic...
Page 528
β-hcg - 2 art:730474-01d rev. Date: 24-jan-17 metrological traceability the i-stat system test for β-hcg measures hcg amount-of-substance concentration in plasma or the plasma fraction of whole blood (dimension iu/l) for in vitro diagnostic use. β-hcg values assigned to abbott point of care’s contro...
Page 529
Rev. Date: 24-jan-17 art:730474-01d β-hcg - 3 then decrease and plateau. β -hcg circulates as the intact molecule in the serum of women who have an uncomplicated pregnancy. The subunits are cleaved rapidly and cleared by the kidneys. 6 with the availability of sensitive quantitative assays for the m...
Page 530
β-hcg - 4 art:730474-01d rev. Date: 24-jan-17 method comparison: i-stat vs abbott architect (iu/l) i-stat (whole blood) vs architect (fresh plasma) i-stat (fresh plasma) vs architect (fresh plasma) n 288 287 slope 1.01 1.00 intercept 2.40 -3.21 sy.X 0.0084 0.0044 syy 5.7% 4.0% sxx n/a 2.4% r 0.990 0...
Page 531
Rev. Date: 24-jan-17 art:730474-01d β-hcg - 5 whole blood sample concentration (iu/l) diluted concentration iu/l % recovery a 104.1 52.9 101.8% b 288.9 132.0 91.4% c 899.8 467.2 103.8% plasma sample concentration (iu/l) diluted concentration iu/l % recovery a 88.8 - - b 298.0 - - c 971.6 - - a+b - 2...
Page 532
β-hcg - 6 art:730474-01d rev. Date: 24-jan-17 interfering substances (such as heterophilic antibodies, non-specific proteins, or hcg-like substances) may falsely depress or falsely elevate results. 18,27,28 these interfering substances may cause false results over the entire range of the assay, not ...
Page 533
Rev. Date: 24-jan-17 art:730474-01d β-hcg - 7 interference testing interference studies were based on clsi guideline ep7-a2. 25 the following substances were found to have no significant effect (less than 10%) on the β-hcg method when added to a plasma pool containing approximately 40 i u/l of β-hcg...
Page 534
β-hcg - 8 art:730474-01d rev. Date: 24-jan-17 references 1. Braunstein gd, vaitukaitis jl, carbone pp, ross gt. Ectopic production of human chorionic gonadotropin by neoplasms. Ann intern med 1973; 78:39-45. 2. Tietz nw, clinical guide to laboratory tests, 4th ed. 2006. P. 2160-2161. 3. Lenton ea, n...
Page 535
Rev. Date: 24-jan-17 art:730474-01d β-hcg - 9 19. Alfthan h, haglund c, dabek j, stenman u-h. Concentrations of human choriogonadotropin, its β-subunit, and the core fragment of the β-subunit in serum and urine of men and nonpregnant women. Clin chem, 1992; 38:1981-7. 20. Borkowski a, muquardt c. Hu...
Page 536
Abbottpointofcareinc. 100and200abbottparkroad abbottpark,il60064•usa emergoeurope molenstraat15 2513bh,thehague thenetherlands ©2017abbottpointofcareinc.Allrightsreserved.Printedinusa. β-hcg - 10 art: 730474-01d rev. Date: 24-jan-17.
Page 537: Technical Bulletin
Technical bulletin i-stat ® abbott point of care inc. • 100 & 200 abbott park road • abbott park, il 60064 • usa art: 730475-01f rev. Date: 14-mar-16 the i-stat total beta-human chorionic gonadotropin (β-hcg) cartridge a. Intended use the i-stat total beta-human chorionic gonadotropin (β-hcg) assay ...
Page 538
Art: 730475-01f rev. Date: 14-mar-16 2 e. Sample volume requirement the i-stat total β-hcg cartridge requires a minimum sample volume of 17 µl to fill. Excess amounts beyond this requirement will not impair the results. However, excess blood or plasma will be present at the inlet of the cartridge an...
Page 539
3 rev. Date: 14-mar-16 art: 730475-01f • detection of very low levels of hcg does not rule out pregnancy. 10 low levels of hcg can occur in apparently healthy, nonpregnant subjects. 11,12 because hcg values double approximately every 48 hours in a normal pregnancy, 10 patients with very low levels o...
Page 540
Art: 730475-01f rev. Date: 14-mar-16 4 • the frequency of suppressed results is affected by atmospheric pressure. Suppressed result rates may increase with higher elevations (decreased barometric pressure) and may become persistent if testing is performed at more than 7500 feet above sea level. Wher...
Page 541
5 rev. Date: 14-mar-16 art: 730475-01f b. Warnings and precautions handle the products using the same safety precautions used when handling any potentially infectious material. The human plasma used in the preparation of these products has been tested by fda approved test methods and found negative/...
Page 542
Art: 730475-01f rev. Date: 14-mar-16 6 i-stat total β-hcg calibration verification kit a. Intended use the i-stat total β-hcg calibration verification materials are used to verify the calibration of the i-stat total β-hcg test throughout the reportable range. B. Warnings and precautions handle the p...
Page 543
7 rev. Date: 14-mar-16 art: 730475-01f always ensure that the lot number and software revision on the value assignment sheet matches the lot number of the vial in use and the software revision in the handheld. Target values are specific to the i-stat system. Results may differ if used with other met...
Page 544
Art: 730475-01f rev. Date: 14-mar-16 8 a. Customizing the handheld to disable or enable the qualitative β-hcg result using the handheld keypad 1. Press to turn on the handheld. 2. Press to change screen to administration menu. 3. Press (customization). 4. Press (change). 5. Press (no password is req...
Page 545
9 rev. Date: 14-mar-16 art: 730475-01f 4. If the location to which this handheld is assigned has a check mark under the use default profile column, double click on the alphanumeric code under preferences in the default customization profile column. Otherwise, double click on the alphanumeric code un...
Page 546
Art: 730475-01f rev. Date: 14-mar-16 10 7. Click ok and answer yes to the question about changing the preferences. 8. Download the handheld(s) to the cds from a downloader in the location to which the handheld is assigned. This action will upload the chosen customization features into the handheld. ...
Page 547
11 rev. Date: 14-mar-16 art: 730475-01f 3. If the location to which this handheld is assigned has a check mark under uses default, under the default customization profile column, double click the alphanumeric code under preferences. Otherwise, double click the alphanumeric code under the preferences...
Page 548
Art: 730475-01f rev. Date: 14-mar-16 12 7. Download the handheld(s) to the i-stat/de from a downloader in the location to which the handheld is assigned. This action will upload the chosen customization features into the handheld. Repeat step 7 for all handhelds from the same location to be customiz...
Page 549
13 rev. Date: 14-mar-16 art: 730475-01f references 1. Braunstein gd, vaitukaitis jl, carbone pp, ross gt. Ectopic production of human chorionic gonadotropin by neoplasms. Ann intern med 1973; 78:39-45. 2. Tietz nw, clinical guide to laboratory tests, 3rd ed. 1995. P. 134-136. 3. Cole la. Background ...
Page 550
Art: 730475-01f rev. Date: 14-mar-16 14 16. Schroff rw, foon ka, beatty sm, oldham rk, morgan jr ac. Human anti murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer res 1985; 45:879-85. 17. Lagrew dc, wilson ea, jawad mj. Determinations of gestational age by seru...
Page 551: Technical Bulletin
Art: 730271-01a rev. Date: 05-mar-12 technical bulletin i-stat ® new ultralife 9-volt lithium battery current ultralife 9-volt lithium battery new ultralife 9-volt lithium battery for use with the i-stat system i-stat is a registered trademark of the abbott group of companies in various jurisdiction...
Page 553
Art: 716625-01c rev. Date: 07-apr-09 replacing the nimh rechargeable battery in the martel printer used with the i-stat ® 1 analyzer background the nimh rechargeable battery contained in the martel printers can be replaced if the printer has a battery door. Refer to figure 1 in appendix. Procedure 1...
Page 554
I-stat is a registered trademark of the abbott group of companies in various jurisdictions. 2 art: 716625-01c rev. Date: 07-apr-09 6. Slide the connector onto the two metal connector pins behind the alignment tab on the printer. Refer to figures 5 and 6 in appendix. 7. Once the wires are connected, ...
Page 555: Technical Bulletin
Art: 714962-01d rev. Date: 10-jun-11 technical bulletin i-stat ® instructions for restoring analyzers that produce *** for hematocrit and quality check code 23 hematocrit star-out (***) results and quality check code 23 may be reduced by restoring an analyzer with the reusable i-stat ceramic conditi...
Page 556
Art: 714962-01d rev. Date: 10-jun-11 2 ceramic conditioning cartridge usage log ceramic cartridge serial number 1 _______________________ new strip check one box for each time the ceramic cartridge is run in the analyzer. Typically, this means two boxes are checked each time an analyzer is restored ...
Page 557
3 art: 714962-01d rev. Date: 10-jun-11 maintaining the ceramic conditioning cartridge the ceramic conditioning cartridge consists of an aluminum base that supports a ceramic “strip.” the strip is a white strip of alumina that is held down by a brass retainer and retainer screw. The ceramic cartridge...
Page 558
Art: 714962-01d rev. Date: 10-jun-11 4 procedure for replacing the strip follow the same procedure as rotating the strip, except discard the old strip and insert a new strip in its place..
Page 559: Technical Bulletin
Art: 714261-01d rev. Date: 10-jan-11 technical bulletin i-stat ® hematocrit determination in the i-stat ® system and comparison to other methods this bulletin describes the four most common methods for determining hematocrit (microhematocrit, conductometric, and as calculated by automated cell count...
Page 560
Art: 714261-01d rev. Date: 10-jan-11 2 conductivity methods systems using the conductivity method, such as the i-stat system, measure the electrical conductance of a whole blood sample. Plasma conducts electrical current, and blood cells act as insulators. A sample with a relatively high hematocrit ...
Page 561
3 art: 714261-01d rev. Date: 10-jan-11 method-dependent / sample-specific component the method-dependent / sample-specific component results from the specific interferences which affect different measurement methods. Each sample has specific characteristics, which may cause different methods to have...
Page 562
Art: 714261-01d rev. Date: 10-jan-11 4 white blood cells interference from white blood cells depends upon the size of the cell. As an example, the hematocrit reading will be increased by 1 %pcv when the white cell count is 50,000 per microliter, assuming the average white blood cell occupies twice t...
Page 563
5 art: 714261-01d rev. Date: 10-jan-11 bibliography standards for microhematocrit determination 1. Clsi. Procedure for determining packed cell volume by the microhematocrit method; approved standard - third edition. Clsi document h7-a3 [isbn 1-56238-413-9]. Clsi, 940 west valley road, suite 1400, wa...
Page 564
Art: 714261-01d rev. Date: 10-jan-11 6 18. Strauchen j, alston w, anderson j, gustafson z, fajardo l: inaccuracy in automated measure- ment of hematocrit and corpuscular indices in the presence of severe hyperglycemia. Blood 57:1065-1067, 1981. 19. Morse ee, kalache g, germino w, stockwell r: increa...
Page 565
7 art: 714261-01d rev. Date: 10-jan-11 37. Rautman e, newbower r: a practical analysis of the electrical conductivity of blood. Ieee trans- actions on biomedical engineering bme 30-3: 141-154, 1983. 38. Riley jb, burgess bm, smith ca, crowley jc, soronen sw: in vitro measurement of the accuracy of a...
Page 566
Art: 714261-01d rev. Date: 10-jan-11 8.
Page 567: Technical Bulletin
Abbott point of care inc. • 100 & 200 abbott park road • abbott park, il 60064 • usa art: 716240-01d rev. Date: 26-feb-16 technical bulletin i-stat ® k 2 edta and k 3 edta customization for hematocrit on the i-stat ® system purpose this technical bulletin contains the information needed to select th...
Page 568
Art: 716240-01d rev. Date: 26-feb-16 2 if the calibration of a comparative method is uncertain, determine the customization setting by minimizing the average bias between methods as follows: • check that the results from hematocrit controls for both i-stat and comparative methods are acceptable. • i...
Page 569
3 rev. Date: 26-feb-16 art: 716240-01d references 1. Bull bs, van assendelft ow, fujimoto k, et al (international council for standardization in haematology expert panel on cytometry). Recommendations for reference method for the packed cell volume (icsh standard 2001). Lab hematol. 7:148-170 (2001)...
Page 570
Art: 716240-01d rev. Date: 26-feb-16 4.
Page 571: Modes For The I-Stat
Art:715617-01c rev.Date:08/23/06 technical bulletin i-stat ® act test result calibration options: prewarmed vs. Non-prewarmed result calibration modes for the i-stat ® 1 analyzer background theactivatedclottingtime(act)testhasbeeninexistenceforover30years.Itisthemostpopulartest formeasuringtheeffect...
Page 572
Art:715617-01c rev.Date:08/23/06 2 i-stat act calibration currently, the i-stat ® celite ® act and i-stat ® kaolin act tests are factory calibrated by mathematically adjusting the raw i-stat “clot time” to match the hemochron ® celite tube result. This calibration is performed by testing cartridges ...
Page 573
3 art:715617-01c rev.Date:08/23/06 representative data effectofsampletubetemperatureonhemochronactresultsusingpairedsamples:prewarmedsample tubesvs.Non-prewarmedsampletubes..
Page 574
Art:715617-01c rev.Date:08/23/06 4 i-stat celite act vs. Room temperature hemochron ftca510: prewarmed (prewrm) vs. Non- prewarmed (nonwrm)calibrationmodes..
Page 575
5 art:715617-01c rev.Date:08/23/06 analyzer display and cds changes duetothenewactresultcalibrationoption,thereareseveralchangestotheanalyzerdisplayaswellasto thecentraldatastation(cds).Majorchangesarenotedbelow: • thei-statpcaandbamarenotcapableofofferingthenewactresultcalibrationoption.All pcaandb...
Page 576
Art:715617-01c rev.Date:08/23/06 6 • the i-stat 1 analyzer is capable of offering both the nonwrm and prewrm act customization settings. These customizations can be viewed, selected and changed via the resultscustomizationsectiononthei-stat1analyzer kaolin act handheld customization. Results customi...
Page 577
7 art:715617-01c rev.Date:08/23/06 limitations and warnings • the nonwrm calibration mode applies to the patient path only, and will not be applied to the control or proficiency testing pathway. Control or proficiency samples run in the patient pathway may produce erroneous results. • different loca...
Page 578
Art:715617-01c rev.Date:08/23/06 8.
Page 579: Technical Bulletin
Abbott point of care inc. • 100 & 200 abbott park road • abbott park, il 60064 • usa art: 716144-01ad rev. Date: 16-jan-17 technical bulletin i-stat ® support services abbott point of care and its distributors are committed to helping you resolve any problems with the i-stat ® system: i-stat 1 handh...
Page 580
Art:716144-01ad rev.Date:16-jan-17 2 kenya phillips healthcare technologies ltd phillips business park mombasa road nairobi, kenya tel.No.: 254-7336-12025 lesotho obsidian health ltd cosmo business park malibongwe drive randburg, south africa 2188 tel.No.: +27 87 3535600 libya al- harameen pharmaceu...
Page 581
3 rev. Date: 16-jan-17 art: 716144-01ad india mayana enterprise 219, shreyas complex opp. Jain derasar, navranpura ahmedabad, gujarat india 380009 tel: +91 079 36421752 india iris healthcare technologies pvt ltd no. 15 2nd street jd durairaj nagar chennai, aminijikarai india 600029 tel: 91 22 660660...
Page 582
Art:716144-01ad rev.Date:16-jan-17 4 beijing huiwen yuanmei technology & trading co., ltd 4d no. 1 building, third department guanglian industrial park,no. 2 kechuang dongwu street, light mechanical and electrical integration base, tongzhou zhongguancun science and technology park tongzhou district,...
Page 583
5 rev. Date: 16-jan-17 art: 716144-01ad azerbaijan albatros heltkeyr 6 yu. Guseinov str. Baku, azerbaijan az1021 tel.No: +994125648635 belarus baltas pharma ou tallinn, harjumaa rae vlad, veldi tee 2 tallinn, 75325 estonia tel. No.: +375296800008 belgium & luxembourg abbott belgium abbott sa/nv aven...
Page 584
Art:716144-01ad rev.Date:16-jan-17 6 russia nearmedic plus llc aviakonstruktora mikoyan 12 moscow, russia 125252 tel. No.: +7 (985) 222-41-87 russia technoproject ltd. Boytsova per, 4 saint petersburg, russia 190068 tel. No.: +78122729787 scotland point of care testing ltd units 1-7 arbroath busines...
Page 585
7 rev. Date: 16-jan-17 art: 716144-01ad latin america antigua american hospital supply (ahs) 1060 maitland center commons maitland, fl usa 32751 tel.: 407 475 1168 argentina drogueria artigas s.A. Av. Jose luis chorroarin, 1079 buenos aires capital federal argentina c1427cxh tel.: +64 3 338 0999 bah...
Page 586
Art:716144-01ad rev.Date:16-jan-17 8 i-stat is a registered trademark of the abbott group of companies in various jurisdictions. Mexico abbott laboratories de mexico universal international service calle 539 n6 uh san juan de argon 07969 mexico tel: +525558097632 paraguay index s.A.C.I. Boqueron no....
Page 587
Art:721106-01a rev.Date:03/03/08 technical bulletin i-stat ® april 2008 update to the i-stat central data station version 5 monitors a new alarm monitor has been added which displays real time alerts and conditions of the system that requireattention.Thisinitialreleaseactivatesasetofalarmsrelatedtoa...
Page 588
Art:721106-01a rev.Date:03/03/08 2 clickingonanindividualalarmcategoryopensawindowdisplayingspecificdetailsofthealarmsdetected inthatcategory,thedate/timetheyoccurred,andinwhatlocation. Selectedalarmdetailsorallalarmdetailsmaybedeletedbyclickingtheacknowledgebuttonatthebottom ofthewindow. A report o...
Page 589
3 art:721106-01a rev.Date:03/03/08 6. Todisableanentirealarmcategory,uncheckthe“enabled”boxnexttothealarmcategoryyou wishtodisable. 7. Todisableaspecificalarmcontainedinanalarmcategory,clickthealarmcategorycontaining the alarm to be disabled, and then uncheck the “enabled” box next to the alarm you ...
Page 590
Art:721106-01a rev.Date:03/03/08 4 i-stat central data station customization a. Results viewer users now have the option to include/exclude the patient name from the trend display or printout in the resultsviewer.Thedefaultsettingistohavethepatientnameincludedinthetrendreport. Toexcludethepatientnam...
Page 591
5 art:721106-01a rev.Date:03/03/08 inventory workspace intheitemstabpage,itemscannowbemovedtothe“availableitems:”listusingadrag/droptechnique. Instrument /location workspace insteadofhighlightinganinstrumentserialnumberandclickingon instrument → move…fromthemenu baror move inst. Inthetoolbar,instrum...
Page 592
Art:721106-01a rev.Date:03/03/08 6 quality check codes viewer anewcolumnhasbeenaddedtothequalitycheckcodesviewerindicatingthequalitycodecategory correspondingtotheindividualqualitycheckcode..
Page 593
Art:722831-01a rev.Date:19-feb-09 technical bulletin i-stat ® april 2009 update to the i-stat central data station version 5 customization workspace anewfeaturehasbeenaddedallowinguserstodeleteunusedpreferencessothattheynolongerappearin the apply preferenceswindow. Note:thedefault0preferencesmaynotb...
Page 594
Art:722831-01a rev.Date:19-feb-09 2 b. Todeleteallunusedpreferences: • clickthedelete all unused preferencesradiobutton. • click delete. • aconfirmationmessagewillappearaskingifyouwanttodeletethepreferences.Click yes todeletethepreferences. Data viewer 1. A new feature has been added to the results ...
Page 595
3 art:722831-01a rev.Date:19-feb-09 forexample,ifauserwantstoseeatrenddisplayorobtainamulti-resultprintoutforaparticularpatientid number,andthispatientidnumberhasrecordsattachedwithionizedcalciumvaluesinbothmmol/land mg/dl,amessagewillappearindicatingthatthedisplay/printoutwillnotbeallowed..
Page 596
Art:722831-01a rev.Date:19-feb-09.
Page 597: Technical Bulletin
Art: 720654-01b rev. Date: 09-aug-13 technical bulletin i-stat ® using i-stat ® analyzer customization features to minimize id entry errors introduction this technical bulletin describes seven i-stat ® analyzer customization features that can help minimize patient and operator id entry errors. Id en...
Page 598
Art: 720654-01b rev. Date: 09-aug-13 2 customization feature technical bulletin section i-stat 1 analyzer i-stat portable clinical analyzer (pca) barcode scanning section 1 x use operator list section 2 x excluding operator ids from analyzer printout section 3 x x set max/min id length section 4 x x...
Page 599
3 art: 720654-01b rev. Date: 09-aug-13 analyzer default choices for id length analyzer type operator id patient id i-stat 1 analyzer 0-15 0-15 i-stat pca 0-7 0-12 once the pocc defines the minimum and maximum limits through customization, the analyzer will then only accept id numbers that contain th...
Page 600
Art: 720654-01b rev. Date: 09-aug-13 4 notes: 1. Except for operator list, all id entry customization features described in this bulletin are available on the i-stat 1 analyzer using: • analyzerkeypadcustomization,or • thei-statcdscustomizationworkspace. Theoperatorlistfeatureisonlyavailablethrought...
Page 601
5 art: 720654-01b rev. Date: 09-aug-13 4. Figure2illustratesthei-statcdscustomizationworkspacemainpage.Theuseoperatorlist feature,discussedinthistechnicalbulletin,ismarkedbythenumberedcircle 2 indicating the section of the bulletin where this feature is described. 2 figure 2 i-statisaregisteredtrade...
Page 602
Art: 720654-01b rev. Date: 09-aug-13 6.
Page 603: Technical Bulletin
Art: 721317-01c rev. Date: 29-aug-12 technical bulletin i-stat ® the i-stat system manufacturer’s quality system instructions overview the manufacturer’s quality system instructions (mqsi) represent what are necessary to ensure quality results (accurate, precise and reliable) based upon the specific...
Page 604
Art: 721317-01c rev. Date: 29-aug-12 2 manufacturer’s quality system instructions (continued) the list below defines the i‑stat system mqsi components. Number mqsi description 4 ensure proper cartridge storage a. Ensure that refrigerator storage conditions for stored cartridges are between 2˚c – 8˚c...
Page 605: Technical Bulletin
Abbott park, il 60064 • usa art: 725703-01a rev. Date: 08-mar-10 technical bulletin i-stat ® updates to the i-stat 1 downloader/re- chargers overview abbott point of care continuously seeks to improve the reliability of our product lines. As such, we have made three (3) changes to our downloader/rec...
Page 606
Art: 725703-01a rev. Date: 08-mar-10 2.